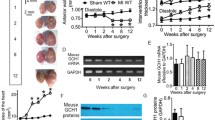
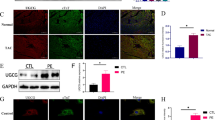
Abstract
Several post-translational modifications figure prominently in ventricular remodeling. The beta-O-linkage of N-acetylglucosamine (O-GlcNAc) to proteins has emerged as an important signal in the cardiovascular system. Although there are limited insights about the regulation of the biosynthetic pathway that gives rise to the O-GlcNAc post-translational modification, much remains to be elucidated regarding the enzymes, such as O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), which regulate the presence/absence of O-GlcNAcylation. Recently, we showed that the transcription factor, E2F1, could negatively regulate OGT and OGA expression in vitro. The present study sought to determine whether E2f1 deletion would improve post-infarct ventricular function by de-repressing expression of OGT and OGA. Male and female mice were subjected to non-reperfused myocardial infarction (MI) and followed for 1 or 4 week. MI significantly increased E2F1 expression. Deletion of E2f1 alone was not sufficient to alter OGT or OGA expression in a naïve setting. Cardiac dysfunction was significantly attenuated at 1-week post-MI in E2f1-ablated mice. During chronic heart failure, E2f1 deletion also attenuated cardiac dysfunction. Despite the improvement in function, OGT and OGA expression was not normalized and protein O-GlcNAcyltion was not changed at 1-week post-MI. OGA expression was significantly upregulated at 4-week post-MI but overall protein O-GlcNAcylation was not changed. As an alternative explanation, we also performed guided transcriptional profiling of predicted targets of E2F1, which indicated potential differences in cardiac metabolism, angiogenesis, and apoptosis. E2f1 ablation increased heart size and preserved remote zone capillary density at 1-week post-MI. During chronic heart failure, cardiomyocytes in the remote zone of E2f1-deleted hearts were larger than wildtype. These data indicate that, overall, E2f1 exerts a deleterious effect on ventricular remodeling. Thus, E2f1 deletion improves ventricular remodeling with limited impact on enzymes regulating O-GlcNAcylation.










Similar content being viewed by others
Abbreviations
- ANP:
-
Atrial natriuretic peptide
- BCL2:
-
B-cell lymphoma 2
- BNP:
-
Brain natriuretic peptide
- CYTB:
-
Cytochrome b
- E2F1:
-
E2F transcription factor 1
- GFPT1:
-
Glutamine fructose-6-phosphate transaminase 1
- GFPT2:
-
Glutamine fructose-6-phosphate transaminase 2
- HBP:
-
Hexosamine biosynthetic pathway
- MI:
-
Myocardial infarction
- NDUFS1:
-
NADH: ubiquinone oxidoreductase core subunit S1
- O-GlcNAc:
-
β-O-Linked N-acetylglucosamine
- OGA:
-
O-GlcNAcase
- OGT:
-
O-GlcNAc transferase
- PDK1:
-
Pyruvate dehydrogenase kinase 1
- PDK4:
-
Pyruvate dehydrogenase kinase 4
- PFKFB1:
-
6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 1
- PFKFB2:
-
6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 1
- PGC1α:
-
Peroxisome proliferator-activated receptor gamma, co-activator 1 alpha
- PGC1β:
-
Peroxisome proliferator-activated receptor gamma, co-activator 1 beta
- VEGFA:
-
Vascular endothelial growth factor A
References
Angelis E, Zhao P, Zhang R, Goldhaber JI, Maclellan WR (2011) The role of E2F-1 and downstream target genes in mediating ischemia/reperfusion injury in vivo. J Mol Cell Cardiol 51:919–926. https://doi.org/10.1016/j.yjmcc.2011.09.012
Brainard RE, Watson LJ, Demartino AM, Brittian KR, Readnower RD, Boakye AA, Zhang D, Hoetker JD, Bhatnagar A, Baba SP, Jones SP (2013) High fat feeding in mice is insufficient to induce cardiac dysfunction and does not exacerbate heart failure. PLoS One 8:e83174. https://doi.org/10.1371/journal.pone.0083174
Champattanachai V, Marchase RB, Chatham JC (2007) Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am J Physiol Cell Physiol 292:C178–C187. https://doi.org/10.1152/ajpcell.00162.2006
Chatham JC, Not LG, Fulop N, Marchase RB (2008) Hexosamine biosynthesis and protein O-glycosylation: the first line of defense against stress, ischemia, and trauma. Shock 29:431–440. https://doi.org/10.1097/shk.0b013e3181598bad
Dassanayaka S, Brainard RE, Watson LJ, Long BW, Brittian KR, DeMartino AM, Aird AL, Gumpert AM, Audam TN, Kilfoil PJ, Muthusamy S, Hamid T, Prabhu SD, Jones SP (2017) Cardiomyocyte Ogt limits ventricular dysfunction in mice following pressure overload without affecting hypertrophy. Basic Res Cardiol 112:23. https://doi.org/10.1007/s00395-017-0612-7
Dassanayaka S, Zheng Y, Gibb AA, Cummins TD, McNally LA, Brittian KR, Jagatheesan G, Audam TN, Long BW, Brainard RE, Jones SP, Hill BG (2018) Cardiac-specific overexpression of aldehyde dehydrogenase 2 exacerbates cardiac remodeling in response to pressure overload. Redox Biol 17:440–449. https://doi.org/10.1016/j.redox.2018.05.016
Doenst T, Nguyen TD, Abel ED (2013) Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res 113:709–724. https://doi.org/10.1161/CIRCRESAHA.113.300376
Fulop N, Feng W, Xing D, He K, Not LG, Brocks CA, Marchase RB, Miller AP, Chatham JC (2008) Aging leads to increased levels of protein O-linked N-acetylglucosamine in heart, aorta, brain and skeletal muscle in Brown-Norway rats. Biogerontology 9:139–151. https://doi.org/10.1007/s10522-007-9123-5
Hart GW (2014) Minireview series on the thirtieth anniversary of research on O-GlcNAcylation of nuclear and cytoplasmic proteins: nutrient regulation of cellular metabolism and physiology by O-GlcNAcylation. J Biol Chem 289:34422–34423. https://doi.org/10.1074/jbc.R114.609776
Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, Opie L (2014) Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 383:1933–1943. https://doi.org/10.1016/S0140-6736(14)60107-0
Jensen RV, Johnsen J, Kristiansen SB, Zachara NE, Botker HE (2013) Ischemic preconditioning increases myocardial O-GlcNAc glycosylation. SCJ, Scand Cardiovasc J. https://doi.org/10.3109/14017431.2012.756984
Jensen RV, Zachara NE, Nielsen PH, Kimose HH, Kristiansen SB, Botker HE (2013) Impact of O-GlcNAc on cardioprotection by remote ischaemic preconditioning in non-diabetic and diabetic patients. Cardiovasc Res 97:369–378. https://doi.org/10.1093/cvr/cvs337
Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, Hart GW, Marban E (2008) Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation 117:1172–1182. https://doi.org/10.1161/CIRCULATIONAHA.107.730515
Lindsey ML, Bolli R, Canty JM Jr, Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE, Heusch G (2018) Guidelines for experimental models of myocardial ischemia and infarction. American journal of physiology. Heart Circ Physiol 314:H812–H838. https://doi.org/10.1152/ajpheart.00335.2017
Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB (2006) Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol 40:303–312. https://doi.org/10.1016/j.yjmcc.2005.11.003
Ma Z, Vosseller K (2014) Cancer metabolism and elevated O-GlcNAc in oncogenic signaling. J Biol Chem 289:34457–34465. https://doi.org/10.1074/jbc.R114.577718
McLarty JL, Marsh SA, Chatham JC (2012) Post-translational protein modification by O-linked N-acetyl-glucosamine: its role in mediating the adverse effects of diabetes on the heart. Life Sci. https://doi.org/10.1016/j.lfs.2012.08.006
Muthusamy S, DeMartino AM, Watson LJ, Brittian KR, Zafir A, Dassanayaka S, Hong KU, Jones SP (2014) MicroRNA-539 is up-regulated in failing heart, and suppresses O-GlcNAcase expression. J Biol Chem 289:29665–29676. https://doi.org/10.1074/jbc.M114.578682
Muthusamy S, Hong KU, Dassanayaka S, Hamid T, Jones SP (2015) E2F1 transcription factor regulates O-linked N-acetylglucosamine (O-GlcNAc) transferase and O-GlcNAcase expression. J Biol Chem 290:31013–31024. https://doi.org/10.1074/jbc.M115.677534
Ngoh GA, Hamid T, Prabhu SD, Jones SP (2009) O-GlcNAc signaling attenuates ER stress-induced cardiomyocyte death. Am J Physiol Heart Circ Physiol 297:H1711–H1719. https://doi.org/10.1152/ajpheart.00553.2009
Park K, Saudek CD, Hart GW (2010) Increased expression of beta-N-acetylglucosaminidase in erythrocytes from individuals with pre-diabetes and diabetes. Diabetes 59:1845–1850. https://doi.org/10.2337/db09-1086
Perez-Cervera Y, Dehennaut V, Aquino Gil M, Guedri K, Solorzano Mata CJ, Olivier-Van Stichelen S, Michalski JC, Foulquier F, Lefebvre T (2013) Insulin signaling controls the expression of O-GlcNAc transferase and its interaction with lipid microdomains. FASEB J 27:3478–3486. https://doi.org/10.1096/fj.12-217984
Sansbury BE, DeMartino AM, Xie Z, Brooks AC, Brainard RE, Watson LJ, DeFilippis AP, Cummins TD, Harbeson MA, Brittian KR, Prabhu SD, Bhatnagar A, Jones SP, Hill BG (2014) Metabolomic analysis of pressure-overloaded and infarcted mouse hearts. Circ Heart Fail 7:634–642. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001151
Slawson C, Copeland RJ, Hart GW (2010) O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci 35:547–555. https://doi.org/10.1016/j.tibs.2010.04.005
Tan EP, McGreal SR, Graw S, Tessman R, Koppel SJ, Dhakal P, Zhang Z, Machacek M, Zachara NE, Koestler DC, Peterson KR, Thyfault JP, Swerdlow RH, Krishnamurthy P, DiTacchio L, Apte U, Slawson C (2017) Sustained O-GlcNAcylation reprograms mitochondrial function to regulate energy metabolism. J Biol Chem 292:14940–14962. https://doi.org/10.1074/jbc.M117.797944
Wang K, An T, Zhou LY, Liu CY, Zhang XJ, Feng C, Li PF (2015) E2F1-regulated miR-30b suppresses cyclophilin D and protects heart from ischemia/reperfusion injury and necrotic cell death. Cell Death Differ 22:743–754. https://doi.org/10.1038/cdd.2014.165
Wang K, Zhou LY, Wang JX, Wang Y, Sun T, Zhao B, Yang YJ, An T, Long B, Li N, Liu CY, Gong Y, Gao JN, Dong YH, Zhang J, Li PF (2015) E2F1-dependent miR-421 regulates mitochondrial fragmentation and myocardial infarction by targeting Pink1. Nat Commun 6:7619. https://doi.org/10.1038/ncomms8619
Watson LJ, Facundo HT, Ngoh GA, Ameen M, Brainard RE, Lemma KM, Long BW, Prabhu SD, Xuan YT, Jones SP (2010) O-Linked beta-N-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci USA 107:17797–17802. https://doi.org/10.1073/pnas.1001907107
Watson LJ, Long BW, DeMartino AM, Brittian KR, Readnower RD, Brainard RE, Cummins TD, Annamalai L, Hill BG, Jones SP (2014) Cardiomyocyte Ogt is essential for postnatal viability. Am J Physiol Heart Circ Physiol 306:H142–H153. https://doi.org/10.1152/ajpheart.00438.2013
Wohlschlaeger J, Schmitz KJ, Takeda A, Takeda N, Vahlhaus C, Stypmann J, Schmid C, Baba HA (2010) Reversible regulation of the retinoblastoma protein/E2F-1 pathway during “reverse cardiac remodelling” after ventricular unloading. J Heart Lung Transplant 29:117–124. https://doi.org/10.1016/j.healun.2009.09.017
Wolfram JA, Liner A, Richardson SL, Zhu X, Smith MA, Hoit BD, Lee HG (2011) The role of E2F1 in the development of hypertrophic cardiomyopathy. Int J Clin Exp Pathol 4:521–525
Wu M, Zhou J, Cheng M, Boriboun C, Biyashev D, Wang H, Mackie A, Thorne T, Chou J, Wu Y, Chen Z, Liu Q, Yan H, Yang Y, Jie C, Tang YL, Zhao TC, Taylor RN, Kishore R, Losordo DW, Qin G (2014) E2F1 suppresses cardiac neovascularization by down-regulating VEGF and PlGF expression. Cardiovasc Res 104:412–422. https://doi.org/10.1093/cvr/cvu222
Wysoczynski M, Dassanayaka S, Zafir A, Ghafghazi S, Long BW, Noble C, DeMartino AM, Brittian KR, Bolli R, Jones SP (2016) A new method to stabilize C-kit expression in reparative cardiac mesenchymal cells. Front Cell Dev Biol 4:78. https://doi.org/10.3389/fcell.2016.00078
Yuzwa SA, Vocadlo DJ (2014) O-GlcNAc and neurodegeneration: biochemical mechanisms and potential roles in Alzheimer’s disease and beyond. Chem Soc Rev 43:6839–6858. https://doi.org/10.1039/c4cs00038b
Zhang Z, Tan EP, VandenHull NJ, Peterson KR, Slawson C (2014) O-GlcNAcase expression is sensitive to changes in O-GlcNAc homeostasis. Front Endocrinol (Lausanne) 5:206. https://doi.org/10.3389/fendo.2014.00206
Acknowledgements
The authors acknowledge the University of Louisville Diabetes and Obesity Center’s Imaging and Physiology Core.
Funding
Dr. Jones has been supported by Grants from the NIH (R01 HL131647, P30 GM127607, and P01 HL078825). Dr. Dassanayaka was supported by an American Heart Association Predoctoral Fellowship—Great Rivers Affiliate (14PRE19710015).
Author information
Authors and Affiliations
Contributions
SD performed experiments and analyzed data; wrote and revised manuscript. KRB performed experiments and analyzed data. AJ performed experiments and analyzed data. LAH performed experiments and analyzed data. TNA performed experiments and analyzed data. BWL performed experiments and analyzed data. LTH performed experiments and analyzed data. DWR analyzed data. GM performed experiments and analyzed data. MGC performed experiments and analyzed data. SU provided samples; revised manuscript. SM performed experiments and analyzed data. AMG performed experiments and analyzed data. SPJ designed experiments; wrote and revised manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
395_2019_737_MOESM2_ESM.pdf
Supp Fig. 1: E2F1 deletion does not improve survival after MI. Survival of E2f1+/+ (n = 21) and E2f1−/− (n = 19) mice 1 week after MI (A). Survival of E2f1+/+ (n = 21) and E2f1−/− mice (n = 18) 4 week after MI (B). A log-rank test was used to determine significance between E2f1+/+ and E2f1−/− groups from Kaplan–Meier survival curves (PDF 39 kb)
395_2019_737_MOESM3_ESM.pdf
Supp Table 1: Attenuation of cardiac dysfunction is limited to male E2f1−/− mice. Male and female E2f1−/− (n = 25) and their E2f1+/+ (n = 26) littermates were subjected to echocardiography 1 week after MI. Indices of cardiac function in E2f1−/− and E2f1+/+ mice were separated by genotype and sex. E2f1−/− male mice preserved end-diastolic volume (EDV, p < 0.0001), end-systolic volume (ESV, p < 0.0001), stroke volume (SV, p < 0.01), ejection fraction (EF, p < 0.0001), left ventricular diastolic inner diameter (LVIDd, p < 0.001), left ventricular systolic inner diameter (LVIDs, p < 0.001), fractional shortening (FS, p < 0.01), and cardiac output (CO, p = 0226). There were no changes in interaction between gender and genotype in heart rate (HR), diastolic or systolic left ventricular anterior wall diameter (LVAWd, LVAWs), and diastolic or systolic left ventricular posterior wall diameter (LVPWd, LVPWs) between groups. A 2-way ANOVA with interaction was performed for genotype and gender followed by a Bonferroni post-test for each parameter of cardiac function (PDF 50 kb)
395_2019_737_MOESM4_ESM.pdf
Supp Fig. 2: Expression of Vegfa remains unaffected in acute and chronic heart failure. Vegfa mRNA expression at 1 and 4 week following MI (A and B, respectively). An unpaired Student’s t test was used to determine significance between E2f1+/+ and E2f1−/− groups (PDF 37 kb)
395_2019_737_MOESM5_ESM.pdf
Supp Table 2: Durable improvement in ventricular function is limited to male E2f1−/− mice. Male and female E2f1−/− (n = 15) and their E2f1+/+ (n = 17) littermates were subjected to echocardiography 4 week after MI. Indices of cardiac function in E2f1−/− and E2f1+/+ mice were separated by genotype and sex. E2f1−/− male mice preserved end-diastolic volume (EDV, p < 0.0001), end-systolic volume (ESV, p < 0.0001), stroke volume (SV, p = 0.0182), ejection fraction (EF, p = 0.0002), left ventricular diastolic inner diameter (LVIDd, p = 0.0006), left ventricular systolic inner diameter (LVIDs, p = 0.0008), fractional shortening (FS, p = 0.0076), left ventricular diastolic posterior wall diameter (LVPWd, p = 0.0467), and left ventricular systolic posterior wall diameter (LVPWs, p = 0.0189). There were no changes in interaction between gender and genotype in heart rate (HR), cardiac output (CO), and diastolic or systolic left ventricular anterior wall diameter (LVAWd, LVAWs). A 2-way ANOVA with interaction was performed for genotype and gender followed by a Bonferroni post-test for each parameter of cardiac function (PDF 50 kb)
Rights and permissions
About this article
Cite this article
Dassanayaka, S., Brittian, K.R., Jurkovic, A. et al. E2f1 deletion attenuates infarct-induced ventricular remodeling without affecting O-GlcNAcylation. Basic Res Cardiol 114, 28 (2019). https://doi.org/10.1007/s00395-019-0737-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-019-0737-y