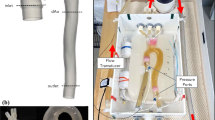
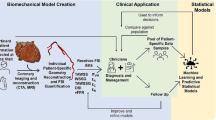
Abstract
Modeling of fluid–structure interactions (FSIs) between the deformable arterial wall and blood flow is necessary to obtain physiologically realistic computational models of cardiovascular systems. However, lack of information on the nature of contact between the outer vessel wall and surrounding tissue presents challenges in prescribing appropriate structural boundary conditions. Imaging techniques used to visualize wall deformation in vivo may be useful for estimating simulation parameters that capture the effects of both vascular composition and surrounding tissue support on the vessel wall displacement. Here, we present a method to calibrate external tissue support parameters in FSI simulations against four-dimensional ultrasound (4DUS) of the murine thoracic aortae. We collected ultrasound, blood pressure, and histological data from several mice infused with angiotensin II (\(n=4\)) and created a representative model of healthy and diseased (at 28 days post-angiotensin II infusion) murine aortae. We ran pulsatile FSI simulations after accounting for increased arterial wall stiffness with varying levels of tissue support, which demonstrated non-trivial variation in not only structural quantities, such as vessel wall deformation, but also hemodynamic quantities, such as wall shear stress across simulations. Furthermore, we compared simulation results with in vivo 4DUS imaging data and observed that the suitable range of the tissue support spring parameter was identical for both healthy and diseased states. This indicated that the same tissue support parameter estimates could be used for modeling the healthy and diseased states of the vessel, provided that changes in arterial wall stiffness had been considered. We anticipate this technique and the tissue support estimates reported herein will help inform computational models of blood flow and vasculature that incorporate the influence of external tissue.
Graphical abstract






Similar content being viewed by others
References
Figueroa CA, Taylor CA, Chiou AJ, Yeh V, Zarins CK (2009) Magnitude and direction of pulsatile displacement forces acting on thoracic aortic endografts. J Endovasc Ther 16(3):350–358. https://doi.org/10.1583/09-2738.1
Bracamonte JH, Wilson JS, Soares JS (2020) Assessing patient-specific mechanical properties of aortic wall and peri-aortic structures from in vivo DENSE magnetic resonance imaging using an inverse finite element method and elastic foundation boundary conditions. J Biomech Eng 142(12):121011. https://doi.org/10.1115/1.4047721
Takizawa K, Bazilevs Y, Tezduyar TE, Long CC, Marsden AL, Schjodt K (2014) ST and ALE-VMS methods for patient-specific cardiovascular fluid mechanics modeling. Math Models Methods Appl Sci 24(12):2437–2486. https://doi.org/10.1142/S0218202514500250
Freidoonimehr N, Chin R, Zander A, Arjomandi M (2022) A review on the effect of temporal geometric variations of the coronary arteries on the wall shear stress and pressure drop. J Biomech Eng 144(12):010801. https://doi.org/10.1115/1.4051923
Elefteriades JA, Sang A, Kuzmik G, Hornick M (2015) Guilt by association: paradigm for detecting a silent killer (thoracic aortic aneurysm). Open Heart 2(1):e000169. https://doi.org/10.1136/openhrt-2014-000169
Campobasso R, Condemi F, Viallon M, Croisille P, Campisi S, Avril S (2018) Evaluation of peak wall stress in an ascending thoracic aortic aneurysm using FSI simulations: effects of aortic stiffness and peripheral resistance. Cardiovasc Eng Technol 9(4):707–722. https://doi.org/10.1007/s13239-018-00385-z
Pons R, Guala A, Rodríguez-Palomares JF, Cajas JC, Dux-Santoy L, Teixidó-Tura G, Molins JJ, Vázquez M, Evangelista A, Martorell J (2020) Fluid–structure interaction simulations outperform computational fluid dynamics in the description of thoracic aorta haemodynamics and in the differentiation of progressive dilation in Marfan syndrome patients. R Soc Open Sci 7(2):191752. https://doi.org/10.1098/rsos.191752
Booher AM, Eagle KA (2011) Diagnosis and management issues in thoracic aortic aneurysm. Am Heart J 162(1):38–46. https://doi.org/10.1016/j.ahj.2011.04.010
Wang Z, Flores N, Lum M, Wisneski AD, Xuan Y, Inman J, Hope MD, Saloner DA, Guccione JM, Ge L, Tseng EE (2021) Wall stress analyses in patients with \(\ge\) 5 cm versus \(<\) 5 cm ascending thoracic aortic aneurysm. J Thorac Cardiovasc Surg 162(5):1452–1459. https://doi.org/10.1016/j.jtcvs.2020.02.046
Cebull HL, Rayz VL, Goergen CJ (2020) Recent advances in biomechanical characterization of thoracic aortic aneurysms. Front Cardiovas Med 7:75. https://doi.org/10.3389/fcvm.2020.00075
Karimi A, Milewicz DM (2016) Structure of the elastin-contractile units in the thoracic aorta and how genes that cause thoracic aortic aneurysms and dissections disrupt this structure. Can J Cardiol 32(1):26–34. https://doi.org/10.1016/j.cjca.2015.11.004
Korenczuk CE, Dhume RY, Liao KK, Barocas VH (2019) Ex vivo mechanical tests and multiscale computational modeling highlight the importance of intramural shear stress in ascending thoracic aortic aneurysms. J Biomech Eng 141(12):121010. https://doi.org/10.1115/1.4045270
Reymond P, Crosetto P, Deparis S, Quarteroni A, Stergiopulos N (2013) Physiological simulation of blood flow in the aorta: comparison of hemodynamic indices as predicted by 3-D FSI, 3-D rigid wall and 1-D models. Med Eng Phys 35(6):784–791. https://doi.org/10.1016/j.medengphy.2012.08.009
Moireau P, Xiao N, Astorino M, Figueroa CA, Chapelle D, Taylor CA, Gerbeau J-F (2012) External tissue support and fluid–structure simulation in blood flows. Biomech Model Mechanobiol 11(1–2):1–18. https://doi.org/10.1007/s10237-011-0289-z
Markl M, Draney MT, Hope MD, Levin JM, Chan FP, Alley MT, Pelc NJ, Herfkens RJ (2004) Time-resolved 3-dimensional velocity mapping in the thoracic aorta. J Comput Assist Tomogr 28(4):459–468. https://doi.org/10.1097/00004728-200407000-00005
Bäumler K, Vedula V, Sailer AM, Seo J, Chiu P, Mistelbauer G, Chan FP, Fischbein MP, Marsden AL, Fleischmann D (2020) Fluid-structure interaction simulations of patient-specific aortic dissection. Biomech Model Mechanobiol 19(5):1607–1628. https://doi.org/10.1007/s10237-020-01294-8
Daugherty A, Rateri DL, Charo IF, Owens AP, Howatt DA, Cassis LA (2010) Angiotensin II infusion promotes ascending aortic aneurysms: attenuation by CCR2 deficiency in apoE–/– mice. Clin Sci 118(11):681–689. https://doi.org/10.1042/CS20090372
Daugherty A, Manning MW, Cassis LA (2000) Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein \(E\)-deficient mice. J Clin Investig 105(11):1605–1612. https://doi.org/10.1172/JCI7818
Acuna A, Berman AG, Damen FW, Meyers BA, Adelsperger AR, Bayer KC, Brindise MC, Bungart B, Kiel AM, Morrison RA, Muskat JC, Wasilczuk KM, Wen Y, Zhang J, Zito P, Goergen CJ (2018) Computational fluid dynamics of vascular disease in animal models. J Biomech Eng 140(8):080801. https://doi.org/10.1115/1.4039678
Adelsperger AR, Phillips EH, Ibriga HS, Craig BA, Green LA, Murphy MP, Goergen CJ (2018) Development and growth trends in angiotensin II-induced murine dissecting abdominal aortic aneurysms. Physiol Rep 6(8):e13668. https://doi.org/10.14814/phy2.13668
Phillips EH, Di Achille P, Bersi MR, Humphrey JD, Goergen CJ (2017) Multi-modality imaging enables detailed hemodynamic simulations in dissecting aneurysms in mice. IEEE Trans Med Imaging 36(6):1297–1305. https://doi.org/10.1109/TMI.2017.2664799
Damen FW, Berman AG, Soepriatna AH, Ellis JM, Buttars SD, Aasa KL, Goergen CJ (2017) High-frequency 4-dimensional ultrasound (4DUS): a reliable method for assessing murine cardiac function. Tomography 3(4):180–187. https://doi.org/10.18383/j.tom.2017.00016
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31(3):1116–1128. https://doi.org/10.1016/j.neuroimage.2006.01.015
Bazilevs Y, Hsu M-C, Benson DJ, Sankaran S, Marsden AL (2009) Computational fluid–structure interaction: methods and application to a total cavopulmonary connection. Comput Mech 45(1):77–89. https://doi.org/10.1007/s00466-009-0419-y
Hsu M-C, Bazilevs Y (2011) Blood vessel tissue prestress modeling for vascular fluid–structure interaction simulation. Finite Elem Anal Des 47(6):593–599. https://doi.org/10.1016/j.finel.2010.12.015
Updegrove A, Wilson NM, Merkow J, Lan H, Marsden AL, Shadden SC (2017) SimVascular: an open source pipeline for cardiovascular simulation. Ann Biomed Eng 45(3):525–541. https://doi.org/10.1007/s10439-016-1762-8
Lan H, Updegrove A, Wilson NM, Maher GD, Shadden SC, Marsden AL (2018) A re-engineered software interface and workflow for the open-source simvascular cardiovascular modeling package. J Biomech Eng 140(2):024501. https://doi.org/10.1115/1.4038751
Vedula V, Lee J, Xu H, Kuo C-CJ, Hsiai TK, Marsden AL (2017) A method to quantify mechanobiologic forces during zebrafish cardiac development using 4-D light sheet imaging and computational modeling. PLoS Comput Biol 13(10):1005828. https://doi.org/10.1371/journal.pcbi.1005828
Bazilevs Y, Calo VM, Hughes TJR, Zhang Y (2008) Isogeometric fluid–structure interaction: theory, algorithms, and computations. Comput Mech 43(1):3–37. https://doi.org/10.1007/s00466-008-0315-x
Esmaily-Moghadam M, Bazilevs Y, Marsden AL (2013) A new preconditioning technique for implicitly coupled multidomain simulations with applications to hemodynamics. Comput Mech 52(5):1141–1152. https://doi.org/10.1007/s00466-013-0868-1
Taylor CA, Hughes TJR, Zarins CK (1998) Finite element modeling of blood flow in arteries. Comput Methods Appl Mech Eng 158(1–2):155–196. https://doi.org/10.1016/S0045-7825(98)80008-X
Soudah E, Ng EYK, Loong TH, Bordone M, Pua U, Narayanan S (2013) CFD modelling of abdominal aortic aneurysm on hemodynamic loads using a realistic geometry with CT. Comput Math Methods Med 2013:1–9. https://doi.org/10.1155/2013/472564
Simo JC, Hughes TJR (1998) Computational inelasticity. Interdisciplinary applied mathematics, vol 7. Springer, New York. https://doi.org/10.1007/b98904
Weiss D, Long A, Tellides G, Avril S, Humphrey J, Bersi M (2022) Evolving mural defects, dilatation, and biomechanical dysfunction in angiotensin II-induced thoracic aortopathies. Arterioscler Thromb Vasc Biol 42(8):973–986. https://doi.org/10.1161/ATVBAHA.122.317394
Bellini C, Bersi MR, Caulk AW, Ferruzzi J, Milewicz DM, Ramirez F, Rifkin DB, Tellides G, Yanagisawa H, Humphrey JD (2017) Comparison of 10 murine models reveals a distinct biomechanical phenotype in thoracic aortic aneurysms. J R Soc Interface 14(130):1–8. https://doi.org/10.1098/rsif.2016.1036
Tezduyar TE, Takizawa K, Brummer T, Chen PR (2011) Space-time fluid–structure interaction modeling of patient-specific cerebral aneurysms. Int J Numer Methods Biomed Eng 27(11):1665–1710. https://doi.org/10.1002/cnm.1433
Tezduyar TE, Sathe S, Schwaab M, Conklin BS (2008) Arterial fluid mechanics modeling with the stabilized space-time fluid–structure interaction technique. Int J Numer Methods Fluids 57(5):601–629. https://doi.org/10.1002/fld.1633
Takizawa K, Tezduyar TE (2014) Fluid–structure interaction modeling of patient-specific cerebral aneurysms. In: Visualization and simulation of complex flows in biomedical engineering. Lecture notes in computational vision and biomechanics, vol 12. Springer, Dordrecht, pp. 25–45. https://doi.org/10.1007/978-94-007-7769-9
Takizawa K, Moorman C, Wright S, Purdue J, McPhail T, Chen PR, Warren J, Tezduyar TE (2011) Patient-specific arterial fluid–structure interaction modeling of cerebral aneurysms. Int J Numer Methods Fluids 65(1–3):308–323. https://doi.org/10.1002/fld.2360
Vignon-Clementel IE, Figueroa CA, Jansen KE, Taylor CA (2010) Outflow boundary conditions for 3D simulations of non-periodic blood flow and pressure fields in deformable arteries. Comput Methods Biomech Biomed Eng 13(5):625–640. https://doi.org/10.1080/10255840903413565
Xiao N, Alastruey J, Figueroa CA (2014) A systematic comparison between 1-D and 3-D hemodynamics in compliant arterial models. Int J Numer Methods Biomed Eng 30(2):204–231. https://doi.org/10.1002/cnm.2598
Olufsen MS (1999) Structured tree outflow condition for blood flow in larger systemic arteries. Am J Phys Heart Circul Phys 276(1):257–268. https://doi.org/10.1152/ajpheart.1999.276.1.H257
Petterson N, Sjoerdsma M, van Sambeek M, van de Vosse F, Lopata R (2021) Mechanical characterization of abdominal aortas using multi-perspective ultrasound imaging. J Mech Behav Biomed Mater 119:104509. https://doi.org/10.1016/j.jmbbm.2021.104509
Durbak E, Tarraf S, Gillespie C, Germano E, Cikach F, Blackstone E, Emerton K, Colbrunn R, Bellini C, Roselli EE (2021) Ex-vivo biaxial load testing analysis of aortic biomechanics demonstrates variation in elastic energy distribution across the aortic zone zero. J Thorac Cardiovasc Surg. https://doi.org/10.1016/j.jtcvs.2021.09.071
Chung J, Hulbert GM (1993) A time integration algorithm for structural dynamics with improved numerical dissipation: the generalized-\(\alpha\) method. J Appl Mech 60(2):371–375. https://doi.org/10.1115/1.2900803
Valente R, Mourato A, Brito M, Xavier J, Tomás A, Avril S (2022) Fluid–structure interaction modeling of ascending thoracic aortic aneurysms in simvascular. Biomechanics 2:189–204. https://doi.org/10.3390/biomechanics2020016
Acknowledgements
This publication was made possible with support from the Indiana Clinical and Translational Sciences Institute which is funded in part by Award No. UL1TR002529 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also graciously acknowledge the assistance of the Purdue University Histology Research Laboratory and computational resources supported by the Rosen Center for Advanced Computing (RCAC) at Purdue University. Additional support was provided to HLC from a Bilsland Fellowship and to CJG from the Leslie A. Geddes Endowment at Purdue University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Goergen is a paid consultant and member of the Scientific Advisory Board for FUJIFILM VisualSonics, Inc. None of the authors have a conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1: Grid independence
To ensure that computational quantities reported, such as pressure, velocity and wall shear stress, were independent of the grid resolution of the fluid domain’s mesh, a grid sensitivity analysis was performed. The pertinent details for each mesh are shown in Table 6. A two-step approach was used to establish grid independence. First, a core mesh resolution was determined such that pressure and velocity were independent of the core mesh resolution. Second, varying degrees of mesh refinement close to the fluid–solid interface were implemented on top of the chosen core mesh resolution from the previous step, to ensure that the computed wall shear stress was independent of the near-wall mesh refinement resolution. In Fig. 6c–f, the area-averaged and point-wise pressure and velocity magnitude at the inlet plane, as well as at an arbitrary point located in the interior of the ascending region of the aorta (see Fig. 6a) are plotted over a single cardiac cycle.
Pressure and velocity data over a cardiac cycle at the inlet plane (panels c and e) and at a point (panels d and f) in the interior of the ascending aorta (shown in a) for different mesh resolutions (shown in b). The error bars of each plot point show a deviation of 5% from the corresponding value on the finest mesh (\(\Delta x = 0.008\,{{\hbox {cm}}}\)). Abbreviations used—\(\vert {\mathbf {v}}\vert\) Velocity magnitude, R Right, L Left, A Anterior, P Posterior. Based on the above plots, \(\Delta x = 0.01\,{\hbox {cm}}\) was chosen as the optimal core mesh resolution
Based on the plots in Fig. 6c–f, we observed that the pressure and velocity magnitude values computed on both the coarse and medium grid (i.e. with \(\Delta x = 0.015\,{\hbox {cm}}\) and \(\Delta x = 0.01\,{\hbox {cm}}\)) lie within a 5% margin of the values computed on the fine grid. However, in Fig. 6d, the velocity magnitude for the coarse grid (\(\Delta x = 0.015\,{\hbox {cm}}\)) lies beyond this tolerance margin. Therefore, \(\Delta x = 0.01\,{\hbox {cm}}\) was determined to be the core mesh resolution of choice.
Next, Fig. 7 shows the x, y, and z components of the WSS (wall shear stress) computed at a point on the surface of the ascending aorta. Here, the core mesh resolution was identical in all cases (\(\Delta x = 0.01\,{\hbox {cm}}\)). However, close to the fluid–solid interface, different number of layers of mesh refinement (0, 3, 4, and 5) were considered (see Fig. 7a) From Fig. 7b–d, we observed a non-trivial difference (\(> 5\%\)) between the surface shear stress values computed on meshes with and without mesh refinement. Furthermore, meshes with different levels of mesh refinement (\(N_{{\mathrm{BL}}}=3,4\), and 5) yield shear stress values within the above tolerance limit with minor differences in the computation time. Therefore, we proceeded with a mesh refinement level of \(N_{{\mathrm{BL}}} = 4\), to balance the need for increased resolution with the corresponding computational cost.
Components (b–d) of the WSS over a cardiac cycle at a point on the interior surface of the ascending aorta (shown by a dot in the model geometry in a), for different number of boundary layers each. \(N_{{\mathrm{BL}}}\) represents the number of layers of boundary layer elements. Here, \(N_{{\mathrm{BL}}} = 0\) represents a mesh without boundary layer refinement. The error bars on each plot point show a deviation of 5% from the corresponding value on the mesh with the largest number of boundary layer refinements (i.e. \(N_{{\mathrm{BL}}} = 5\)). Based on the above plots, the boundary layer mesh resolution corresponding to \(N_{{\mathrm{BL}}} = 4\) was chosen as for the FSI simulations. Abbreviations used—R Right, L Left, S Superior, I Inferior
A constant time step of \(\Delta t=10^{-5}\,{\hbox {s}}\) was used for all cases. Table 6 reports an estimate of the maximum cell-based Courant number computed for each of the meshes used, over a single cardiac cycle. The Courant number was computed as:
where \(\vert {\mathbf {v}}\vert\) is the velocity magnitude at the cell center, \(\Delta t\) is the time step size, and \(\Delta x\) is a length scale computed for each cell as \(\Delta x = \mathcal {V}^{1/3}\), where \(\mathcal {V}\) is the cell volume.
We observed that, for cases for which the maximum \({{\mathrm{CFL}}} > 1\), only a few cells (\({<} \,5\)) outside the region of interest (viz. the ascending aorta) exceeded the threshold. This observation, together with the fact that the time integration scheme implemented in svFSI is an implicit scheme [45], allowed us to use the same time step size of \(\Delta t=10^{-5}\,{\hbox {s}}\) for the subsequent FSI simulations as well.
Appendix 2: Material properties
The Young’s moduli for the Day 0 and 28 time points were estimated using circumferential stress-stretch data for wildtype C57BL/6J and AngII-infused apolipoprotein E\(^{-/-}\) mice, respectively, as reported by Bellini et al. [35]. For a biaxial state of stress of an incompressible neo-Hookean material, the theoretical relationship between circumferential stress \(\sigma _{\theta \theta }\) and circumferential stretch ratio \(\lambda _{\theta \theta }\) is:
where p is the Lagrange multiplier that enforces the incompressibility constraint. Therefore, using the biaxial stress-stretch data reported in [35], the Young’s modulus was estimated to be three times the slope of the best fit line to \(\sigma _{\theta \theta }\) versus \(\lambda _{\theta \theta }\) (see Fig. 8). The values are reported in Table 2.
Experimental circumferential stress-stretch-squared data from Bellini et al. [35] along with best fit lines and corresponding best fit equations (with units implied). The Young’s modulus (in kPa) was estimated to be three times the fitted slope (colour figure online)
Appendix 3: Comparison of other cross-sections
This appendix provides plots of the effective diameter and non-overlapping area (see Sect. 4.1) at the other two cross-sections for the Day 0 and Day 28 time points (Figs. 9 and 10). Overall, the observations regarding these cross-sections are consistent with the data obtained for the cross-section reported in Sect. 4.1.
Quantitative metrics comparing segmentations from 4DUS and FSI simulations for different values of k at peak systole. a, b Show the location of the cross-section being considered. Red squares in c, d show the plot of effective diameter of the cross-section, obtained from FSI simulations (calculated using Eq. (12)) as a function of tissue support parameter k. The solid red line represents the effective diameter of the same cross-section obtained from segmentations of 4DUS imaging data. e, f Show the variation of non-overlapping area at the cross-section, calculated using Eq. (13) as a function of the varying tissue support parameter k (colour figure online)
Quantitative metrics comparing segmentations from 4DUS and FSI simulations for different values of k at peak systole. a, b Show the location of the cross-section being considered. Red squares in c, d show the plot of effective diameter of the cross-section, obtained from FSI simulations (calculated using Eq. (12)) as a function of tissue support parameter k. The solid red line represents the effective diameter of the same cross-section obtained from segmentations of 4DUS imaging data. e, f Show the variation of non-overlapping area at the cross-section, calculated using Eq. (13) as a function of the varying tissue support parameter k (colour figure online)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shidhore, T.C., Cebull, H.L., Madden, M.C. et al. Estimating external tissue support parameters with fluid–structure interaction models from 4D ultrasound of murine thoracic aortae. Engineering with Computers 38, 4005–4022 (2022). https://doi.org/10.1007/s00366-022-01735-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00366-022-01735-1