Abstract
Objectives
To assess the diagnostic accuracy of nerve thickening on MRI to predict early-stage postlaminar optic nerve invasion (PLONI) in retinoblastoma. Furthermore, this study aimed to incorporate measurements into a multiparametric model for radiological determination of PLONI.
Methods
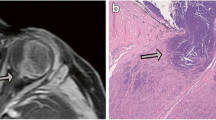
In this retrospective multicenter case–control study, high-spatial-resolution 3D T2-weighted MR images were used to measure the distal optic nerve. Histopathology was the reference standard for PLONI. Two neuroradiologists independently measured the optic nerve width, height, and surface at 0, 3, and 5 mm from the most distal part of the optic nerve. Subsequently, PLONI was scored on contrast-enhanced T1-weighted and 3D T2-weighted images, blinded for clinical data. Optic nerve measurements with the highest diagnostic accuracy for PLONI were incorporated into a prediction model for radiological determination of PLONI.
Results
One hundred twenty-four retinoblastoma patients (median age, 22 months [range, 0–113], 58 female) were included, resulting in 25 retinoblastoma eyes with histopathologically proven PLONI and 206 without PLONI. ROC analysis of axial optic nerve width measured at 0 mm yielded the best area under the curve of 0.88 (95% confidence interval: 0.79, 0.96; p < 0.001). The optimal width cutoff was ≥ 2.215 mm, with a sensitivity of 84% (95% CI: 64, 95%) and specificity of 83% (95% CI: 75, 89%) for detecting PLONI. Combining width measurements with the suspicion of PLONI on MRI sequences resulted in a prediction model with an improved sensitivity and specificity of respectively up to 88% and 92%.
Conclusion
Postlaminar optic nerve thickening can predict early-stage postlaminar optic nerve invasion in retinoblastoma.
Clinical relevance statement
This study provides an additional tool for clinicians to help determine postlaminar optic nerve invasion, which is a risk factor for developing metastatic disease in retinoblastoma patients.
Key Points
• The diagnostic accuracy of contrast-enhanced MRI for detecting postlaminar optic nerve invasion is limited in retinoblastoma patients.
• Optic nerve thickening can predict postlaminar optic nerve invasion.
• A prediction model combining MRI features has a high sensitivity and specificity for detecting postlaminar optic nerve invasion.




Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- CSF:
-
Cerebrospinal fluid
- ICC:
-
Intraclass correlation coefficient
- IQR:
-
Interquartile ranges
- MRI:
-
Magnetic resonance imaging
- PLONI:
-
Postlaminar optic nerve invasion
- ROC:
-
Receiver operating characteristic
References
Dimaras H, Kimani K, Dimba EA et al (2012) Retinoblastoma Lancet 379:1436–1446
Finger PT, Harbour JW, Karcioglu ZA (2002) Risk factors for metastasis in retinoblastoma. Surv Ophthalmol 47:1–16
Lu JE, Francis JH, Dunkel IJ et al (2018) Metastases and death rates after primary enucleation of unilateral retinoblastoma in the USA 2007–2017. Br J Ophthalmol. https://doi.org/10.1136/bjophthalmol-2018-312915
Kaliki S, Shields CL, Rojanaporn D et al (2013) High-risk retinoblastoma based on international classification of retinoblastoma: analysis of 519 enucleated eyes. Ophthalmology 120:997–1003
Bosaleh A, Sampor C, Solernou V et al (2012) Outcome of children with retinoblastoma and isolated choroidal invasion. Arch Ophthalmol 130:724–729
Dimaras H, Corson TW, Cobrinik D et al (2015) Retinoblastoma Nat Rev Dis Primers 1:15021
Munier FL, Beck-Popovic M, Chantada GL et al (2019) Conservative management of retinoblastoma: Challenging orthodoxy without compromising the state of metastatic grace. “Alive, with good vision and no comorbidity”. Prog Retin Eye Res 73:100764. https://doi.org/10.1016/j.preteyeres.2019.05.005
Mallipatna ACGB, Chéves-Barrios P (2017) AJCC Cancer Staging Manual. In: Amin MB, Edge SB, Greene FL et al (eds) Springer, New York, pp 819–831
Choucair ML, Brisse HJ, Fréneaux P et al (2020) Management of advanced uni- or bilateral retinoblastoma with macroscopic optic nerve invasion. Pediatr Blood Cancer 67:e27998
de Jong MC, de Graaf P, Noij DP et al (2014) Diagnostic performance of magnetic resonance imaging and computed tomography for advanced retinoblastoma: a systematic review and meta-analysis. Ophthalmology 121:1109–1118
de Graaf P, Barkhof F, Moll AC et al (2005) Retinoblastoma: MR imaging parameters in detection of tumor extent. Radiology 235:197–207
Brisse HJ, de Graaf P, Galluzzi P et al (2015) Assessment of early-stage optic nerve invasion in retinoblastoma using high-resolution 1.5 Tesla MRI with surface coils: a multicentre, prospective accuracy study with histopathological correlation. Eur Radiol 25:1443–1452
Jansen RW, van der Heide S, Cardoen L et al (2022) MRI can reliably differentiate optic nerve inflammation from tumor invasion in retinoblastoma with orbital cellulitis. Ophthalmology. https://doi.org/10.1016/j.ophtha.2022.06.013
De Jong MC, van der Meer FJ, Goricke SL et al (2016) Diagnostic accuracy of intraocular tumor size measured with MR imaging in the prediction of postlaminar optic nerve invasion and massive choroidal invasion of retinoblastoma. Radiology 279:817–826
de Jong MC (2017) Epidemiology and imaging of retinoblastoma (Doctoral dissertation, Vrije Universiteit Amsterdam). Retrieved from https://hdl.handle.net/1871/55441
de Jong MC, Van Der Valk P, Jansen RW et al (2020) Full-width postlaminar optic nerve tumor invasion of retinoblastoma as risk-factor for leptomeningeal spread of retinoblastoma. A case report and review of the literature. Ophthalmic Genet 41:69–72
Bossuyt PM, Reitsma JB, Bruns DE et al (2003) The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med 138:W1-12
de Graaf P, Goricke S, Rodjan F et al (2012) Guidelines for imaging retinoblastoma: imaging principles and MRI standardization. Pediatr Radiol 42:2–14
Koo TK, Li MY (2016) A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 15:155–163
Bohning D, Holling H, Patilea V (2011) A limitation of the diagnostic-odds ratio in determining an optimal cut-off value for a continuous diagnostic test. Stat Methods Med Res 20:541–550
de Jong MC, Kors WA, de Graaf P, Castelijns JA, Kivela T, Moll AC (2014) Trilateral retinoblastoma: a systematic review and meta-analysis. Lancet Oncol 15:1157–1167
Sirin S, Schlamann M, Metz KA et al (2015) High-resolution MRI using orbit surface coils for the evaluation of metastatic risk factors in 143 children with retinoblastoma: part 1: MRI vs. histopathology. Neuroradiology 57:805–814
Cho SJ, Kim JH, Baik SH, Sunwoo L, Bae YJ, Choi BS (2021) Diagnostic performance of MRI of post-laminar optic nerve invasion detection in retinoblastoma: a systematic review and meta-analysis. Neuroradiology 63:499–509
Gizewski ER, Wanke I, Jurklies C, Güngör AR, Forsting M (2005) T1 Gd-enhanced compared with CISS sequences in retinoblastoma: superiority of T1 sequences in evaluation of tumour extension. Neuroradiology 47:56–61
Grimes DA, Schulz KF (2005) Compared to what? Finding controls for case-control studies. Lancet 365:1429–1433
Moll AC, Kuik DJ, Bouter LM et al (1997) Incidence and survival of retinoblastoma in The Netherlands: a register based study 1862–1995. Br J Ophthalmol 81:559–562
Brisse HJ, Guesmi M, Aerts I et al (2007) Relevance of CT and MRI in retinoblastoma for the diagnosis of postlaminar invasion with normal-size optic nerve: a retrospective study of 150 patients with histological comparison. Pediatr Radiol 37:649–656
Abramson DH, Schefler AC, Almeida D, Folberg R (2003) Optic nerve tissue shrinkage during pathologic processing after enucleation for retinoblastoma. Arch Ophthalmol 121:73–75
Yuan W, Beaulieu-Jones BK, Yu KH et al (2021) Temporal bias in case-control design: preventing reliable predictions of the future. Nat Commun 12:1107
Acknowledgements
For the European Retinoblastoma Imaging Collaboration
Funding
This research was funded by the Hanarth Foundation, Grant for project titled MRI-based Deep Learning Segmentation and Quantitative Radiomics in Retinoblastoma: A Next Step Towards Personalized Interventions. The funding sources had no influence on data collection, analysis, manuscript preparation or publication.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Marcus C. de Jong.
Conflict of interest
No conflicting relationship exists for any author.
Statistics and biometry
Marcus C. de Jong has significant statistical expertise and oversaw this process.
Informed consent
Written informed consent was waived by the Institutional Review Board (IRB number IRB00002991).
Ethical approval
Institutional Review Board approval was obtained (Institutional review board of the Amsterdam UMC).
Study subjects or cohorts overlap
Some study subjects have been previously reported in “Magnetic Resonance Imaging Can Reliably Differentiate Optic Nerve Inflammation from Tumor Invasion in Retinoblastoma with Orbital Cellulitis” by R.W. Jansen et al (2022) https://doi.org/10.1016/j.ophtha.2022.06.013.
Methodology
• retrospective
• case–control study
• multicenter study
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Bloeme, C.M., Jansen, R.W., Göricke, S. et al. Optic nerve thickening on high-spatial-resolution MRI predicts early-stage postlaminar optic nerve invasion in retinoblastoma. Eur Radiol (2023). https://doi.org/10.1007/s00330-023-10471-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-023-10471-z