Abstract
Objectives
To investigate a comprehensive segmentation of chest X-ray (CXR) in promoting deep learning–based World Health Organization’s (WHO) radiologically confirmed pneumonia diagnosis in children.
Methods
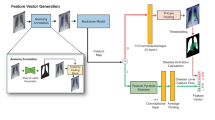
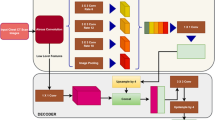
A total of 4400 participants between January 2016 and June 2021were identified for a cross-sectional study and divided into primary endpoint pneumonia (PEP), other infiltrates, and normal groups according to WHO’s diagnostic criteria. The CXR was divided into six segments of left lung, right lung, mediastinum, diaphragm, ext-left lung, and ext-right lung by adopting the RA-UNet. To demonstrate the benefits of lung field segmentation in pneumonia diagnosis, the segmented images and images that were not segmented, which constituted seven segmentation combinations, were fed into the CBAM-ResNet under a three-category classification comparison. The interpretability of the CBAM-ResNet for pneumonia diagnosis was also performed by adopting a Grad-CAM module.
Results
The RA-UNet achieved a high spatial overlap between manual and automatic segmentation (averaged DSC = 0.9639). The CBAM-ResNet when fed with the six segments achieved superior three-category diagnosis performance (accuracy = 0.8243) over other segmentation combinations and deep learning models under comparison, which was increased by around 6% in accuracy, precision, specificity, sensitivity, F1-score, and around 3% in AUC. The Grad-CAM could capture the pneumonia lesions more accurately, generating a more interpretable visualization and enhancing the superiority and reliability of our study in assisting pediatric pneumonia diagnosis.
Conclusions
The comprehensive segmentation of CXR could improve deep learning–based pneumonia diagnosis in childhood with a more reasonable WHO’s radiological standardized pneumonia classification instead of conventional dichotomous bacterial pneumonia and viral pneumonia.
Clinical relevance statement
The comprehensive segmentation of chest X-ray improves deep learning–based WHO confirmed pneumonia diagnosis in children, laying a strong foundation for the potential inclusion of computer-aided pediatric CXR readings in precise classification of pneumonia and PCV vaccine trials efficacy in children.
Key Points
• The chest X-ray was comprehensively segmented into six anatomical structures of left lung, right lung, mediastinum, diaphragm, ext-left lung, and ext-right lung.
• The comprehensive segmentation improved the three-category classification of primary endpoint pneumonia, other infiltrates, and normal with an increase by around 6% in accuracy, precision, specificity, sensitivity, F1-score, and around 3% in AUC.
• The comprehensive segmentation gave rise to a more accurate and interpretable visualization results in capturing the pneumonia lesions.






Similar content being viewed by others
Abbreviations
- 2DAN:
-
2D attention network
- AUC:
-
Area under receiver operating characteristic curve
- CBAM:
-
Convolutional block attention module
- COM:
-
Combination
- CXR:
-
Chest X-ray
- DC-UNet:
-
Dual channel U-Net
- DR:
-
Digital radiography
- DSC:
-
Dice similarity coefficient
- ext-left lung:
-
Extensive left lung area
- ext-right lung:
-
Extensive right lung area
- FN:
-
False negative
- FOV:
-
Field-of-view
- FP:
-
False positive
- Grad-CAM:
-
Gradient-weighted Class Activation Mapping
- PCV:
-
Pneumococcal conjugate vaccine
- PEP:
-
Primary endpoint pneumonia
- RAN:
-
Residual attention network
- RA-UNet:
-
Residual attention-UNet
- ResNet:
-
Residual neural network
- ResNet-50:
-
Residual network with 50 layers
- ResUNet:
-
Residual U-Net
- TN:
-
True negative
- TP:
-
True positive
- TransUNet:
-
Transformer-based U-Net
- WHO:
-
World Health Organization
References
Black RE, Cousens S, Johnson HL et al (2010) Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375:1969–1987
Zhao B, Guo Y, Zheng C et al (2019) Using deep-learning techniques for pulmonary-thoracic segmentations and improvement of pneumonia diagnosis in pediatric chest radiographs. Pediatr Pulmonol 54:1617–1626
Hwang S, Park S (2017) Accurate lung segmentation via network-wise training of convolutional networks. Deep Learning in Medical lmage Analysis and Multimodal learning for Clinical Decision Support. Springer, pp 92–99
Mansoor A, Cerrolaza JJ, Perez G et al (2019) A generic approach to lung field segmentation from chest radiographs using deep space and shape learning. IEEE Trans Biomed Eng 67:1206–1220
Garin M, Carballo DF, Montet R (2012) High discordance of chest x-ray and CT for detection of pulmonary opacities in ED patients: implications for diagnosing pneumonia. Am J Respir Crit Care Med 31:10.1164
Ferreira JR, Cardenas DAC, Moreno RA, de Sá Rebelo MdF, Krieger JE, Gutierrez MA (2020) Multi-view ensemble convolutional neural network to improve classification of pneumonia in low contrast chest x-ray images2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). IEEE, pp 1238–1241
Longjiang E, Zhao B, Liu H et al (2021) Image-based deep learning in diagnosing the etiology of pneumonia on pediatric chest X-rays. Pediatric Pulmonol 56:1036–1044
Mohammed I, Singh N, Venkatasubramanian M (2019) Computer-assisted detection and diagnosis of pediatric pneumonia in chest X-ray images. Available via https://www.patterncomputer.com/wpcontent/uploads/2022/02/Computer-Assisted-Detection-and-Diagnosis-of-Pediatric-Pneumonia-in-Chest-X-ray-Images.pdf
Beadling C, Slifka MK (2004) How do viral infections predispose patients to bacterial infections? Curr Opin Infect Dis 17:185–191
Pavia AT (2013) What is the role of respiratory viruses in community-acquired pneumonia?: What is the best therapy for influenza and other viral causes of community-acquired pneumonia? Infect Dis Clin 27:157–175
Bosch AA, Biesbroek G, Trzcinski K, Sanders EA, Bogaert D (2013) Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog 9:e1003057
Cherian T, Mulholland EK, Carlin JB et al (2005) Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ 83:353–359
Fernandes V, Junior GB, de Paiva AC, Silva AC, Gattass M (2021) Bayesian convolutional neural network estimation for pediatric pneumonia detection and diagnosis. Comput Methods Programs Biomed 208:106259
Selvaraju RR, Cogswell M, Das A, Vedantam R, Parikh D, Batra D (2017) Grad-cam: Visual explanations from deep networks via gradient-based localizationProceedings of the IEEE international conference on computer vision, pp 618–626
Jin Q, Meng Z, Sun C, Cui H, Su R (2020) RA-UNet: a hybrid deep attention-aware network to extract liver and tumor in CT scans. Front Bioeng Biotechnol 8:1471
Woo S, Park J, Lee J-Y, Kweon IS (2018) Cbam: convolutional block attention module. Proceedings of the European conference on computer vision (ECCV), pp 3–19
He K, Zhang X, Ren S, Sun J (2016) Deep residual learning for image recognition. Proceedings of the IEEE conference on computer vision and pattern recognition, pp 770–778
Chen J, Lu Y, Yu Q et al (2021) Transunet: transformers make strong encoders for medical image segmentation. arXiv preprint arXiv:210204306
Zhang Z, Liu Q, Wang Y (2018) Road extraction by deep residual u-net. IEEE Geosci Remote Sens Lett 15:749–753
Lou A, Guan S, Loew M (2021) DC-UNet: rethinking the U-Net architecture with dual channel efficient CNN for medical image segmentation Medical Imaging 2021: Image Processing. SPIE, pp 758–768
Wang F, Jiang M, Qian C et al (2017) Residual attention network for image classification. Proceedings of the IEEE conference on computer vision and pattern recognition, pp 3156–3164
Jin D, Zhou B, Han Y et al (2020) Generalizable, reproducible, and neuroscientifically interpretable imaging biomarkers for Alzheimer’s disease. Adv Sci 7:2000675
Piryonesi SM, El-Diraby TE (2020) Data analytics in asset management: cost-effective prediction of the pavement condition index. J Infrastruct Syst 26:04019036
Ibrahim AU, Ozsoz M, Serte S, Al-Turjman F, Yakoi PS (2021) Pneumonia classification using deep learning from chest X-ray images during COVID-19. Cogn Comput 4:1–13
Zhang X, Han L, Sobeih T et al (2021) CXR-Net: an encoder-decoder-encoder multitask deep neural network for explainable and accurate diagnosis of COVID-19 pneumonia with chest X-ray Images. arXiv preprint arXiv:211010813
Tiwari A, Sharan TS, Sharma S, Sharma N (2022) Deep learning-based automated multiclass classification of chest X-rays into Covid-19, normal, bacterial pneumonia and viral pneumonia. Cogent Eng 9:2105559
Mahomed N, van Ginneken B, Philipsen RH et al (2020) Computer-aided diagnosis for World Health Organization-defined chest radiograph primary-endpoint pneumonia in children. Pediatr Radiol 50:482–491
Chen Y, Roberts CS, Ou W et al (2021) Deep learning for classification of pediatric chest radiographs by WHO’s standardized methodology. PLoS one 16:e0253239
Yang Z, Xu Q, Bao S, Cao X, Huang Q (2021) Learning with multiclass AUC: theory and algorithms. IEEE Trans Pattern Anal Mach Intell 44:7747–7763
Van Calster B, Van Belle V, Condous G, Bourne T, Timmerman D, Van Huffel S (2008) Multi-class AUC metrics and weighted alternatives2008 IEEE International Joint Conference on Neural Networks (IEEE World Congress on Computational Intelligence). IEEE, pp 1390–1396
Gimeno P, Mingote V, Ortega A, Miguel A, Lleida E (2021) Generalizing AUC optimization to multiclass classification for audio segmentation with limited training data. IEEE Signal Process Lett 28:1135–1139
Nishino M, Ashiku SK, Kocher ON, Thurer RL, Boiselle PM, Hatabu H (2006) The thymus: a comprehensive review. Radiographics 26:335–348
Nikolić MZ, Sun D, Rawlins EL (2018) Human lung development: recent progress and new challenges. Development 145:dev163485
Goldstein AJ, Oliva I, Honarpisheh H, Rubinowitz A (2015) A tour of the thymus: a review of thymic lesions with radiologic and pathologic correlation. Can Assoc Radiol J 66:5–15
Funding
This study has received funding by the National Natural Science Foundation of China (grant number 61802330, 61802331) and Xiamen Science and Technology Plan Project (grant number 3502Z202009220).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Qiang Zheng from Yantai University.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
The scientific ethics committee of local hospital approved this retrospective study and waived the requirement for informed patient consent (Approval number: [2022] No.18).
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
The study subjects were collected from the children aged 3–6 years between January 2016 and June 2021 at local children’s hospital.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Zhang, L., Yu, H. et al. A comprehensive segmentation of chest X-ray improves deep learning–based WHO radiologically confirmed pneumonia diagnosis in children. Eur Radiol 34, 3471–3482 (2024). https://doi.org/10.1007/s00330-023-10367-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10367-y