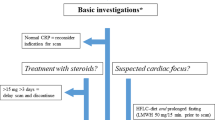
Abstract
Introduction
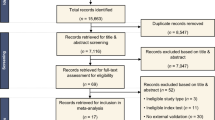
A systematic review and meta-analysis were performed to determine the diagnostic performance of dynamic contrast–enhanced computed tomography (DCE-CT) for the differentiation between malignant and benign pulmonary nodules.
Methods
Ovid MEDLINE and EMBASE were searched for studies published up to October 2018 on the diagnostic accuracy of DCE-CT for the characterisation of pulmonary nodules. For the index test, studies with a minimum of a pre- and post-contrast computed tomography scan were evaluated. Studies with a reference standard of biopsy for malignancy, and biopsy or 2-year follow-up for benign disease were included. Study bias was assessed using QUADAS-2 (Quality Assessment of Diagnostic Accuracy Studies). The sensitivities, specificities, and diagnostic odds ratios were determined along with 95% confidence intervals (CIs) using a bivariate random effects model.
Results
Twenty-three studies were included, including 2397 study participants with 2514 nodules of which 55.3% were malignant (1389/2514). The pooled accuracy results were sensitivity 94.8% (95% CI 91.5; 96.9), specificity 75.5% (69.4; 80.6), and diagnostic odds ratio 56.6 (24.2–88.9). QUADAS 2 assessment showed intermediate/high risk of bias in a large proportion of the studies (52–78% across the domains). No difference was present in sensitivity or specificity between subgroups when studies were split based on CT technique, sample size, nodule size, or publication date.
Conclusion
DCE-CT has a high diagnostic accuracy for the diagnosis of pulmonary nodules although study quality was indeterminate in a large number of cases.
Key Points
• The pooled accuracy results were sensitivity 95.1% and specificity 73.8% although individual studies showed wide ranges of values.
• This is comparable to the results of previous meta-analyses of PET/CT (positron emission tomography/computed tomography) diagnostic accuracy for the diagnosis of solitary pulmonary nodules.
• Robust direct comparative accuracy and cost-effectiveness studies are warranted to determine the optimal use of DCE-CT and PET/CT in the diagnosis of SPNs.





Similar content being viewed by others
Abbreviations
- DCE-CT:
-
Dynamic contrast–enhanced computed tomography
- DOR:
-
Diagnostic odds ratio
- HU:
-
Hounsfield units
- NLR:
-
Negative likelihood ratio
- PET:
-
Positron emission tomography
- PLR:
-
Positive likelihood ratio
- QUADAS:
-
Quality Assessment of Diagnostic Accuracy Studies
- SPN:
-
Solitary pulmonary nodules
- SROC:
-
Summary receiver operator characteristic
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65:5–29. https://doi.org/10.3322/caac.21254
Tanner NT, Dai L, Bade BC, Gebregziabher M, Silvestri GA (2017) Assessing the generalizability of the national lung screening trial: comparison of patients with stage 1 disease. Am J Respir Crit Care Med 196:602–608. https://doi.org/10.1164/rccm.201705-0914OC
Barnett PG, Ananth L, Gould MK (2010) Cost and outcomes of patients with solitary pulmonary nodules managed with PET scans. Chest 137:53–59. https://doi.org/10.1378/chest.08-0529
National Lung Screening Trial Research Team, Aberle DR, Adams AM et al (2011) Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 365:395–409. https://doi.org/10.1056/NEJMoa1102873
MacMahon H, Austin JHM, Gamsu G et al (2005) Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner society. Radiology 237:395–400. https://doi.org/10.1148/radiol.2372041887
MacMahon H, Naidich DP, Goo JM et al (2017) Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner society 2017. Radiology 284:228–243. https://doi.org/10.1148/radiol.2017161659
Callister MEJ, Baldwin DR, Akram AR et al (2015) British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 70(Suppl 2):ii1–ii54. https://doi.org/10.1136/thoraxjnl-2015-207168
Matsumoto M, Koike S, Kashima S, Awai K (2015) Geographic distribution of CT, MRI and PET devices in Japan: a longitudinal analysis based on national census data. PLoS One 10:1–12. https://doi.org/10.1371/journal.pone.0126036
Parker L, Levin DC, Frangos A, Rao VM (2010) Geographic variation in the utilization of noninvasive diagnostic imaging: national Medicare data, 1998-2007. AJR Am J Roentgenol 194:1034–1039. https://doi.org/10.2214/AJR.09.3528
Ohno Y, Nishio M, Koyama H et al (2015) Solitary pulmonary nodules: comparison of dynamic first-pass contrast-enhanced perfusion area-detector CT, dynamic first-pass contrast-enhanced MR imaging, and FDG PET/CT. Radiology 274:563–575. https://doi.org/10.1148/radiol.14132289
Yi CA, Lee KS, Kim EA et al (2004) Solitary pulmonary nodules: dynamic enhanced multi–detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology 233:191–199. https://doi.org/10.1148/radiol.2331031535
Bai RJ1, Cheng XG, Qu H, Shen BZ, Han MJ, Wu ZH (2009) Solitary pulmonary nodules: comparison of multi-slice computed tomography perfusion study with vascular endothelial growth factor and microvessel density. Chin Med J (Engl) 122:541–547. https://doi.org/10.3760/cma.j.issn.0366-6999.2009.05.011
Cronin P, Dwamena BA, Kelly AM, Carlos RC (2008) Solitary pulmonary nodules: meta-analytic comparison of cross-sectional imaging modalities for diagnosis of malignancy. Radiology 246:772–782. https://doi.org/10.1148/radiol.2463062148
McInnes MDF, Moher D, Thombs BD et al (2018) Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA 319:388–396. https://doi.org/10.1001/jama.2017.19163
Whiting PF, Rutjes AWS, Westwood ME et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536. https://doi.org/10.7326/0003-4819-155-8-201110180-00009
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67. https://doi.org/10.18637/jss.v067.i01
Harbord RM, Egger M, Sterne JAC (2006) A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 25:3443–3457. https://doi.org/10.1002/sim.2380
Swensen SJ, Morin RL, Schueler BA et al (1992) Solitary pulmonary nodule: CT evaluation of enhancement with iodinated contrast material - a preliminary report. Radiology 182:343–347
Swensen SJ, Brown LR, Colby TV, Weaver AL (1995) Pulmonary nodules: CT evaluation of enhancement with iodinated contrast material. Radiology 194(2):393–398
Yamashita K, Matsunobe S, Tsuda T et al (1995) Solitary pulmonary nodule: preliminary study of evaluation with incremental dynamic CT. Radiology 194:399–405. https://doi.org/10.1148/radiology.194.2.7824717
Swensen SJ, Brown LR, Colby TV, Weaver AL, Midthun DE (1996) Lung nodule enhancement at CT: prospective findings. Radiology 201:447–455. https://doi.org/10.1148/radiology.201.2.8888239
Potente G, Iacari V, Caimi M (1997) The challenge of solitary pulmonary nodules: HRCT evaluation. Comput Med Imaging Graph 21:39–46
Zhang M, Kono M (1997) Solitary pulmonary nodules: evaluation of blood flow patterns with dynamic CT. Radiology 205:471–478. https://doi.org/10.1148/radiology.205.2.9356631
Swensen SJ, Viggiano RW, Midthun DE et al (2000) Lung nodule enhancement at CT: multicenter study. Radiology 214:73–80. https://doi.org/10.1148/radiology.214.1.r00ja1473
Kim JH, Kim HJ, Lee KH, Kim KH, Lee HL (2004) Solitary pulmonary nodules: a comparative study evaluated with contrast-enhanced dynamic MR imaging and CT. J Comput Assist Tomogr 28:766–775
Orlacchio A, Schillaci O, Antonelli L et al (2007) Nodulo polmonare solitario: Caratterizzazione morfologico-metabolica mediante imaging integrato TCms/FDG-PET. Radiol Med 112:157–173. https://doi.org/10.1007/s11547-007-0132-x
Lee KS, Yi CA, Jeong SY et al (2007) Solid or partly solid solitary pulmonary nodules: their characterization using contrast wash-in and morphologic features at helical CT. Chest 131:1516–1525. https://doi.org/10.1378/chest.06-2526
Ohno Y, Koyama H, Takenaka D et al (2008) Dynamic MRI, dynamic multidetector-row computed tomography (MDCT), and coregistered 2-[fluorine-18]-fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET)/CT: comparative study of capability for management of pulmonary nodules. J Magn Reson Imaging 27:1284–1295. https://doi.org/10.1002/jmri.21348
Choi EJ, Jin GY, Han YM, Lee YS, Kweon KS (2008) Solitary pulmonary nodule on helical dynamic CT scans: analysis of the enhancement patterns using a computer-aided diagnosis (CAD) system. Korean J Radiol 9:401–408. https://doi.org/10.3348/kjr.2008.9.5.401
Bayraktaroglu S, Savaş R, Basoglu ÖK et al (2008) Dynamic computed tomography in solitary pulmonary nodules. J Comput Assist Tomogr 32:222–227. https://doi.org/10.1097/RCT.0b013e318136e29d
Jiang NC, Han P, Zhou CK, Zheng JL, Shi HS, Xiao J (2009) Dynamic enhancement patterns of solitary pulmonary nodules at multi-detector row CT and correlation with vascular endothelial growth factor and microvessel density. Ai Zheng 28:164–169
Dabrowska M, Zukowska M, Krenke R et al (2010) Simplified method of dynamic contrast-enhanced computed tomography in the evaluation of indeterminate pulmonary nodules. Respiration 79:91–96. https://doi.org/10.1159/000213760
Li Y, Yang ZG, Chen TW, Yu JQ, Sun JY, Chen HJ (2010) First-pass perfusion imaging of solitary pulmonary nodules with 64-detector row CT: comparison of perfusion parameters of malignant and benign lesions. Br J Radiol 83:785–790. https://doi.org/10.1259/bjr/58020866
Ohno Y, Koyama H, Matsumoto K et al (2011) Differentiation of malignant and benign pulmonary nodules with quantitative first-pass 320-detector row perfusion CT versus FDG PET/CT. Radiology 258:599–609. https://doi.org/10.1148/radiol.10100245
Ohno Y, Nishio M, Koyama H et al (2013) Comparison of quantitatively analyzed dynamic area-detector CT using various mathematic methods with FDG PET/CT in management of solitary pulmonary nodules. AJR Am J Roentgenol 200:593–602. https://doi.org/10.2214/AJR.12.9197
Shu SJ, Liu BL, Jiang HJ (2013) Optimization of the scanning technique and diagnosis of pulmonary nodules with first-pass 64-detector-row perfusion VCT. Clin Imaging 37:256–264. https://doi.org/10.1016/j.clinimag.2012.05.004
Ribeiro SM, Ruiz RL, Yoo HH, Cataneo DC, Cataneo AJ (2013) Proposal to utilize simplified swensen protocol in diagnosis of isolated pulmonary nodule. Acta Radiol 54:757–764. https://doi.org/10.1177/0284185113481695
Ye XD, Ye JD, Yuan Z, Li WT, Xiao XS (2014) Dynamic CT of solitary pulmonary nodules: comparison of contrast medium distribution characteristic of malignant and benign lesions. Clin Transl Oncol 16:49–56. https://doi.org/10.1007/s12094-013-1039-8
Li ZZ, Huang YL, Song HJ, Wang YJ, Huang Y (2018) The value of 18 F-FDG-PET/CT in the diagnosis of solitary pulmonary nodules: a meta-analysis. Medicine (Baltimore) 97. https://doi.org/10.1097/MD.0000000000010130
Yi CA, Lee KS, Kim B-T et al (2006) Tissue characterization of solitary pulmonary nodule: comparative study between helical dynamic CT and integrated PET/CT. J Nucl Med 47:443–450
Basso Dias A, Zanon M, Altmayer S et al (2019) Fluorine 18-FDG PET/CT and diffusion-weighted MRI for malignant versus benign pulmonary lesions: a meta-analysis. Radiology 290:525–534. https://doi.org/10.1148/radiol.2018181159
Zhou SC, Wang YJ, Ai T et al (2019) Diagnosis of solitary pulmonary lesions with intravoxel incoherent motion diffusion-weighted MRI and semi-quantitative dynamic contrast-enhanced MRI. Clin Radiol 74:409.e7–409.e16. https://doi.org/10.1016/j.crad.2018.12.022
Ducharme J, Goertzen AL, Patterson J, Demeter S (2009) Practical aspects of 18 F-FDG PET when receiving 18 F-FDG from a distant supplier. J Nucl Med Technol 37:164–170. https://doi.org/10.2967/jnmt.109.062950
Comber LA, Keith CJ, Griffiths M, Miles KA (2003) Solitary pulmonary nodules: impact of quantitative contrast-enhanced CT on the cost-effectiveness of FDG-PET. Clin Radiol 58:706–711. https://doi.org/10.1016/S0009-9260(03)00166-1
Church TR, Black WC, Aberle DR et al (2013) Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 368:1980–1991. https://doi.org/10.1056/NEJMoa1209120
Horeweg N, van Rosmalen J, Heuvelmans MA et al (2014) Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol 15:1332–1341. https://doi.org/10.1016/S1470-2045(14)70389-4
Leeflang MM, Rutjes AW, Reitsma JB, Hooft L, Bossuyt PM (2013) Variation of a test’s sensitivity and specificity with disease prevalence. CMAJ 185:537–544. https://doi.org/10.1503/cmaj.121286
Disclaimer
The views expressed are those of the authors and not necessarily those of the NHS, NIHR, or Department of Health.
Funding
The study was funded by NIHR Health Technology Assessment programme, project number 09 22 117 and supported by the NIHR Cambridge Biomedical Research Centre. AC is part-funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care North West Coast (NIHR CLAHRC NWC). FJG is part-funded by an NIHR Senior Investigator award. RCR is part-funded by Cambridge Biomedical Research Centre and Cambridge Cancer Centre. NRQ is part-funded by Cambridge BRC. The funders had no role in the design, analysis, or write-up of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is FJG.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was not required for this study because it is a meta-analysis of published anonymous data.
Ethical approval
Institutional Review Board approval was not required because it is a meta-analysis of published anonymous data.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in the respective original scientific articles from which the data has been extracted by meta-analysis.
Methodology
• Meta-analysis
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 25.5 kb)
Rights and permissions
About this article
Cite this article
Weir-McCall, J.R., Joyce, S., Clegg, A. et al. Dynamic contrast–enhanced computed tomography for the diagnosis of solitary pulmonary nodules: a systematic review and meta-analysis. Eur Radiol 30, 3310–3323 (2020). https://doi.org/10.1007/s00330-020-06661-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06661-8