Abstract
Objective
Histopathological examination (HPE) is the current gold standard for assessing chemotherapy response to tumor, but it is possible only after surgery. The purpose of the study was to develop a noninvasive, imaging-based robust method to delineate, visualize, and quantify the proportions of necrosis and viable tissue present within the tumor along with peritumoral edema before and after neoadjuvant chemotherapy (NACT) and to evaluate treatment response with correlation to HPE necrosis after surgery.
Methods
The MRI dataset of 30 patients (N = 30; male:female = 24:6; age = 17.6 ± 2.7 years) with osteosarcoma was acquired using 1.5 T Philips Achieva MRI scanner before (baseline) and after 3 cycles of NACT (follow-up). After NACT, all patients underwent surgical resection followed by HPE. Simple linear iterative clustering supervoxels and Otsu multithresholding were combined to develop the proposed method—SLICs+MTh—to subsegment and quantify viable and nonviable regions within tumor using multiparametric MRI. Manually drawn ground-truth ROIs and SLICs+MTh-based segmentation of tumor, edema, and necrosis were compared using Jacquard index (JI), Dice coefficient (DC), precision (P), and recall (R). Postcontrast T1W images (PC-T1W) were used to validate the SLICs+MTh-based necrosis. SLICs+MTh-based necrosis volume at follow-up was compared with HPE necrosis using paired t test (p ≤ 0.05).
Results
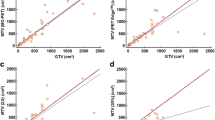
Active tumor, necrosis, and edema were segmented with moderate to satisfactory accuracy (JI = 62–78%; DC = 72–87%; P = 67–87%; R = 63–88%). Qualitatively and quantitatively (DC = 74 ± 9%), the SLICs+MTh-based necrosis area correlated well with the hypointense necrosis areas in PC-T1W. No significant difference (paired t test, p = 0.26; Bland–Altman plot, bias = 2.47) between SLICs+MTh-based necrosis at follow-up and HPE necrosis was observed.
Conclusion
The proposed multiparametric MRI-based SLICs+MTh method performs noninvasive assessment of NACT response in osteosarcoma that may improve cancer treatment monitoring, planning, and overall prognosis.
Key Points
• The simple linear iterative clustering supervoxels and Otsu multithresholding-based technique (SLICs+MTh) successfully estimates the proportion of necrosis, viable tumor, and edema in osteosarcoma in the course of chemotherapy.
• The proposed technique is noninvasive and uses multiparametric MRI to measure necrosis as an indication of anticancer treatment response.
• SLICs+MTh-based necrosis was in satisfactory agreement with histological necrosis after surgery.






Similar content being viewed by others
Abbreviations
- DC:
-
Dice coefficient
- DWI800 :
-
Diffusion weighting factor of 800 s/mm2
- HPE:
-
Histopathological examination
- JI:
-
Jacquard index
- NACT:
-
Neoadjuvant chemotherapy
- NR:
-
Non responder
- P:
-
Precision
- PR:
-
Partial responder
- R:
-
Recall
- SLICs+MTh:
-
Simple linear iterative clustering supervoxels and Otsu multithresholding
- T2W-fatsat:
-
T2-weighted with fat saturation
References
Landis SH, Murray T, Bolden S, Wingo PA (1999) Cancer statistics, 1999. CA Cancer J Clin 49:8–31. https://doi.org/10.3322/canjclin.49.1.8
Bacci G, Picci P, Ferrari S et al (1993) Primary chemotherapy and delayed surgery for nonmetastatic osteosarcoma of the extremities. Results in 164 patients preoperatively treated with high doses of methotrexate followed by cisplatin and doxorubicin. Cancer 72:3227–3238. https://doi.org/10.1002/1097-0142(19931201)72:11<3227::AID-CNCR2820721116>3.0.CO;2-C
Bacci G, Briccoli A, Ferrari S et al (2001) Neoadjuvant chemotherapy for osteosarcoma of the extremity: long-term results of the Rizzoli’s 4th protocol. Eur J Cancer 37:2030–2039. https://doi.org/10.1016/S0959-8049(01)00229-5
Wittig JC, Bickels J, Priebat D et al (2002) Osteosarcoma: a multidisciplinary approach to diagnosis and treatment. Am Fam Physician 65:1123–1132. https://doi.org/10.1111/j.1529-8019.2010.01352.x
Wang C, Du L, Si M et al (2013) Noninvasive assessment of response to neoadjuvant chemotherapy in osteosarcoma of long bones with diffusion-weighted imaging: an initial in vivo study. PLoS One 8:e72679. https://doi.org/10.1371/journal.pone.0072679
Tatum JL, Gillies R, Arbeit JM et al (2006) Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol 82:699–757. https://doi.org/10.1080/09553000601002324
Rejniak KA, Lloyd MC, Reed DR, Bui MM (2015) Diagnostic assessment of osteosarcoma chemoresistance based on virtual clinical trials. Med Hypotheses 85:348–354. https://doi.org/10.1016/j.mehy.2015.06.015
Arunachalam HB, Mishra R, Armaselu B et al (2017) Computer aided image segmentation and classification for viable and non-viable tumor identification in osteosarcoma. Pac Symp Biocomput 22:195–206. https://doi.org/10.1142/9789813207813_0020
Mishra R, Daescu O, Leavey P, et al (2017) Histopathological diagnosis for viable and non-viable tumor prediction for osteosarcoma using convolutional neural network. In: Cai Z, Daescu O, Li M (eds) Bioinformatics research and applications. ISBRA 2017. Lecture notes in computer science. Springer, Cham
Ayala A, Zomosa J (1983) Primary bone tumors: percutaneous needle biopsy. Radiologic-pathologic study of 222 biopsies. Radiology 149:675–679. https://doi.org/10.1148/radiology.149.3.6580673
Brisse H, Ollivier L, Edeline V et al (2004) Imaging of malignant tumours of the long bones in children: monitoring response to neoadjuvant chemotherapy and preoperative assessment. Pediatr Radiol 34:595–605. https://doi.org/10.1007/s00247-004-1192-x
Leung JC, Dalinka MK (2000) Magnetic resonance imaging in primary bone tumors. Semin Roentgenol 35:297–305. https://doi.org/10.1053/sroe.2000.7340
Fletcher BD (1991) Response of osteosarcoma and Ewing sarcoma to chemotherapy: imaging evaluation. AJR Am J Roentgenol 157:825–833. https://doi.org/10.2214/ajr.157.4.1892044
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Hylton N (2007) Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J Clin Oncol 24:3293–3298. https://doi.org/10.1200/JCO.2006.06.8080
Iima M, Le Bihan D (2016) Clinical intravoxel incoherent motion and diffusion MR imaging: past, present, and future. Radiology 278:13–32. https://doi.org/10.1148/radiol.2015150244
Harry VN, Semple SI, Parkin DE, Gilbert FJ (2010) Use of new imaging techniques to predict tumour response to therapy. Lancet Oncol 11:92–102. https://doi.org/10.1016/S1470-2045(09)70190-1
Deng J, Wang Y (2017) Quantitative magnetic resonance imaging biomarkers in oncological clinical trials: current techniques and standardization challenges. Chronic Dis Transl Med 3:8–20. https://doi.org/10.1016/j.cdtm.2017.02.002
Monsky W, Jin B, Molloy C et al (2012) Semi-automated volumetric quantification of tumor necrosis in soft tissue sarcoma using contrast enhanced MRI. Anticancer Res 32:4951–4961
Monsky WL, Garza AS, Kim I et al (2011) Treatment planning and volumetric response assessment for yttrium-90 radioembolization: semiautomated determination of liver volume and volume of tumor necrosis in patients with hepatic malignancy. Cardiovasc Intervent Radiol 34:306–318. https://doi.org/10.1007/s00270-010-9938-3
Ranga A, Agarwal Y, Garg KJ (2017) Gadolinium based contrast agents in current practice: risks of accumulation and toxicity in patients with normal renal function. Indian J Radiol Imaging 27:141–147. https://doi.org/10.4103/0971-3026.209212
Grobner T (2006) Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 21:1104–1108. https://doi.org/10.1093/ndt/gfk062
Koh DM, Collins DJ (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 188:1622–1635. https://doi.org/10.2214/AJR.06.1403
Achanta R, Shaji A, Smith K, Lucchi A, Fua P, Süsstrunk S (2012) SLIC superpixels compared to state-of-the-art superpixel methods. IEEE Trans Pattern Anal Mach Intell 34:2274–2281. https://doi.org/10.1109/TPAMI.2012.120
Otsu N (1979) A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern 9:62–66. https://doi.org/10.1109/TSMC.1979.4310076
ESMO/European Sarcoma Network Working Group (2012) Bone sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23:vii100–vii109. https://doi.org/10.1093/annonc/mds254
Salzer-Kuntschik M, Brand G, Delling G (1983) Determination of the Degree of Morphological Regression Following Chemotherapy in Malignant Bone Tumors. Pathologie 4:135–141
Verma K, Kumar Singh B, Thokec AS (2015) An enhancement in adaptive median filter for edge preservation. Procedia Comput Sci 48:29–36. https://doi.org/10.1016/j.procs.2015.04.106
Sredhar K, Panlal B (2012) Enhancement of images using morphological transformations. Int J Comput Sci Inf Technol 4:33–50. https://doi.org/10.5121/ijcsit.2012.4103
Soltaninejad M, Yang G, Lambrou T et al (2017) Automated brain tumour detection and segmentation using superpixel-based extremely randomized trees in FLAIR MRI. Int J CARS 12:183–203. https://doi.org/10.1007/s11548-016-1483-3
Adjei PE, Nunoo-mensah H, Agbesi RJA, Ndjanzoue JRY (2018) Brain tumor segmentation using SLIC superpixels and optimized thresholding algorithm. Int J Comput Appl 181:1–5. https://doi.org/10.5120/ijca2018917915
Liao X, Zhao J, Jiao C, Lei L, Qiang Y, Cui Q (2016) A segmentation method for lung parenchyma image sequences based on superpixels and a self-generating neural forest. PLoS One 11:e0160556. https://doi.org/10.1371/journal.pone.0160556
Tian Z, Liu L, Zhang Z, Fei B (2017) Superpixel-based segmentation for 3D prostate MR images. IEEE Trans Med Imaging 35:791–801. https://doi.org/10.1109/TMI.2015.2496296
Nishio M, Kono AK, Kubo K, Koyama H, Nishii T, Sugimura K (2015) Tumor segmentation on 18F FDG-PET images using graph cut and local spatial information. Open J Med Imaging 5:174–181. https://doi.org/10.4236/ojmi.2015.53022
Conze P, Noblet V, Rousseau F, Heitz F, Memeo R, Pessaux P (2016) Random forests on hierarchical multi-scale supervoxels for liver tumor segmentation in dynamic contrast-enhanced CT scans. International symposium on biomedical imaging (ISBI). pp 416–419
Just N (2014) Improving tumour heterogeneity MRI assessment with histograms. Br J Cancer 111:2205–2213. https://doi.org/10.1038/bjc.2014.512
Costelloe CM, Raymond AK, Fitzgerald NE et al (2010) Tumor necrosis in osteosarcoma: inclusion of the point of greatest metabolic activity from F-18 FDG PET / CT in the histopathologic analysis. Skeletal Radiol 39:131–140. https://doi.org/10.1007/s00256-009-0785-8
Young H, Baum R, Cremerius U et al (1999) Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. Eur J Cancer 35:1773–1782
Raymond A, Chawla S, Carrasco C et al (1987) Osteosarcoma chemotherapy effect: a prognostic factor. Semin Diagn Pathol 4:212–236
Acknowledgments
The authors would like to thank and acknowledge the valuable input of the intern, Sneha Patil, in data processing and various stages of implementation of the proposed algorithm. The authors would also like to acknowledge the support from Dr. Thilaka Muthiah for copyediting the manuscript for grammar, language, and clarity and the cooperation from MRI technicians Mr. Lalit Gupta and Mr. Udit Kumar during MRI data acquisition.
Funding
This study has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Amit Mehndiratta, Assistant Professor, Centre for Biomedical Engineering, Indian Institute of Technology, Delhi, India, and Department of Biomedical Engineering, All India Institute of Medical Sciences, Delhi, India.
Conflict of interest
The authors declare that they have no conflict of interest.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained with reference number: IEC-103/05.02.2016, RP-26/2016. Approving body: Institute Ethics Committee, Room No.-102, First-Floor, Old O.T.Block, All India Institute of Medical Sciences, Ansari Nagar, New Delhi-110,029, India.
Methodology
• Prospective
• Diagnostic or prognostic study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 14236 kb)
Rights and permissions
About this article
Cite this article
Baidya Kayal, E., Kandasamy, D., Sharma, R. et al. SLIC-supervoxels-based response evaluation of osteosarcoma treated with neoadjuvant chemotherapy using multi-parametric MR imaging. Eur Radiol 30, 3125–3136 (2020). https://doi.org/10.1007/s00330-019-06647-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06647-1