Abstract
Purpose
To determine the diagnostic performance of intravoxel incoherent motion (IVIM) parameters to predict tumor recurrence after hepatectomy in patients with hepatitis B virus (HBV)–related hepatocellular carcinoma (HCC).
Materials and methods
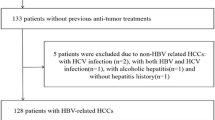
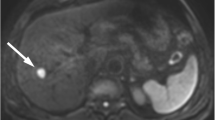
One hundred and fifty-seven patients (mean age 52.54 ± 11.32 years, 87% male) with surgically and pathologically confirmed HCC were included. Regions of interests were drawn including the tumors by two independent radiologists. ADC and IVIM-derived parameters (true diffusion coefficient [D]; pseudodiffusion coefficient [D*]; pseudodiffusion fraction [f]) were obtained preoperatively. The Cox proportional hazards model was used to analyze the predictors associated with tumor recurrence after hepatectomy.
Results
Forty-seven of 157 (29.9%) patients experienced tumor recurrence. The multivariate Cox proportional hazards model revealed that a D value < 0.985 × 10−3 mm2/s (hazard ratio (HR), 0.190; p = 0.023) was a risk factor for tumor recurrence. Additional risk factors included younger age (HR, 0.328; p = 0.034) and higher serum alpha-fetoprotein (AFP) level (HR, 2.079; p = 0.013). Further, receiver operating characteristic (ROC) analysis showed that the area under the curve (AUC) of the obtained Cox regression model improved from 0.68 for the combination of AFP and age alone to 0.724 for the combination of D value, AFP, and age.
Conclusion
The D value derived from the IVIM model is a potential biomarker for the preoperative prediction of recurrence after hepatectomy in patients with HCC. When combined with age and AFP levels, D can improve the predictive performance for tumor recurrence.
Key Points
• The recurrence rate of HCC after hepatectomy was higher in patients with ADC, D, and f values that were lower than the optimal cutoff values.
• The optimal cutoff values of ADC, D, D*, and f for predicting recurrence in HBV associated HCC were 0.858 × 10−3 mm2/s, 0.985 × 10−3 mm2/s, 12.5 × 10−3 mm2/s, and 23.4%, respectively.
• The D value derived from IVIM diffusion-weighted imaging may be a useful biomarker for preoperative prediction of recurrence after hepatectomy in patients with HCC. When combined with age and AFP levels, D can improve the predictive performance for tumor recurrence.





Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AFP:
-
Alpha-fetoprotein
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- D :
-
True diffusion coefficient
- D*:
-
Pseudodiffusion coefficient
- f :
-
Pseudodiffusion fraction
- HBP:
-
Hepatobiliary phase
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HR:
-
Hazard ratio
- ICC:
-
Intra-class correlation coefficient
- IVIM:
-
Intravoxel incoherent motion
- ROC:
-
Receiver operating characteristic
- T2WI:
-
T2-weighted imaging
References
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology 53:1020–1022
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Portolani N, Coniglio A, Ghidoni S et al (2006) Early and late recurrence after liver resection for hepatocellular carcinoma. Ann Surg 243:229–235
Chong CC, Lee KF, Ip PC et al (2012) Pre-operative predictors of post-hepatectomy recurrence of hepatocellular carcinoma: can we predict earlier? Surgeon 10:260–266
Cheng Z, Yang P, Qu S et al (2015) Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB (Oxford) 17:422–427
Li T, Qin LX, Gong X et al (2014) Clinical characteristics, outcome, and risk factors for early and late intrahepatic recurrence of female patients after curative resection of hepatocellular carcinoma. Surgery 156:651–660
Lee S, Kim SH, Lee JE, Sinn DH, Park CK (2017) Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol 67:526–534
An C, Kim DW, Park YN, Chung YE, Rhee H, Kim MJ (2015) Single hepatocellular carcinoma: preoperative MR imaging to predict early recurrence after curative resection. Radiology 276:433–443
Ariizumi S, Kitagawa K, Kotera Y et al (2011) A non-smooth tumor margin in the hepatobiliary phase of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging predicts microscopic portal vein invasion, intrahepatic metastasis, and early recurrence after hepatectomy in patients with hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 18:575–585
Ahn SY, Lee JM, Joo I et al (2015) Prediction of microvascular invasion of hepatocellular carcinoma using gadoxetic acid-enhanced MR and (18)F-FDG PET/CT. Abdom Imaging 40:843–851
Choi JW, Lee JM, Kim SJ et al (2013) Hepatocellular carcinoma: imaging patterns on gadoxetic acid-enhanced MR images and their value as an imaging biomarker. Radiology 267:776–786
Toyoda H, Kumada T, Tada T et al (2013) Non-hypervascular hypointense nodules detected by Gd-EOB-DTPA-enhanced MRI are a risk factor for recurrence of HCC after hepatectomy. J Hepatol 58:1174–1180
Barral M, Taouli B, Guiu B et al (2015) Diffusion-weighted MR imaging of the pancreas: current status and recommendations. Radiology 274:45–63
Muhi A, Ichikawa T, Motosugi U et al (2013) Diffusion-weighted imaging of hepatocellular carcinoma for predicting early recurrence and survival after hepatectomy. Hepatol Int 7:662–668
Xu P, Zeng M, Liu K, Shan Y, Xu C, Lin J (2014) Microvascular invasion in small hepatocellular carcinoma: is it predictable with preoperative diffusion-weighted imaging? J Gastroenterol Hepatol 29:330–336
Suh YJ, Kim MJ, Choi JY, Park MS, Kim KW (2012) Preoperative prediction of the microvascular invasion of hepatocellular carcinoma with diffusion-weighted imaging. Liver Transpl 18:1171–1178
Luciani A, Vignaud A, Cavet M et al (2008) Liver cirrhosis: intravoxel incoherent motion MR imaging--pilot study. Radiology 249:891–899
Yoon JH, Lee JM, Yu MH, Kiefer B, Han JK, Choi BI (2014) Evaluation of hepatic focal lesions using diffusion-weighted MR imaging: comparison of apparent diffusion coefficient and intravoxel incoherent motion-derived parameters. J Magn Reson Imaging 39:276–285
Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168:497–505
Woo S, Lee JM, Yoon JH, Joo I, Han JK, Choi BI (2014) Intravoxel incoherent motion diffusion-weighted MR imaging of hepatocellular carcinoma: correlation with enhancement degree and histologic grade. Radiology 270:758–767
Roayaie S, Jibara G, Taouli B, Schwartz M (2013) Resection of hepatocellular carcinoma with macroscopic vascular invasion. Ann Surg Oncol 20:3754–3760
Gao F, Zhu HK, Zhu YB et al (2016) Predictive value of tumor markers in patients with recurrent hepatocellular carcinoma in different vascular invasion pattern. Hepatobiliary Pancreat Dis Int 15:371–377
Shah SA, Cleary SP, Wei AC et al (2007) Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 141:330–339
Lauwers GY, Terris B, Balis UJ et al (2002) Prognostic histologic indicators of curatively resected hepatocellular carcinomas: a multi-institutional analysis of 425 patients with definition of a histologic prognostic index. Am J Surg Pathol 26:25–34
Verslype C, Rosmorduc O, Rougier P (2012) Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 23 Suppl 7:vii41–48
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology 53:1020–1022
Wurnig MC, Donati OF, Ulbrich E et al (2015) Systematic analysis of the intravoxel incoherent motion threshold separating perfusion and diffusion effects: proposal of a standardized algorithm. Magn Reson Med 74:1414–1422
Sigmund EE, Vivier PH, Sui D et al (2012) Intravoxel incoherent motion and diffusion-tensor imaging in renal tissue under hydration and furosemide flow challenges. Radiology 263:758–769
Patel J, Sigmund EE, Rusinek H, Oei M, Babb JS, Taouli B (2010) Diagnosis of cirrhosis with intravoxel incoherent motion diffusion MRI and dynamic contrast-enhanced MRI alone and in combination: preliminary experience. J Magn Reson Imaging 31:589–600
Kim SY, Lee SS, Byun JH et al (2010) Malignant hepatic tumors: short-term reproducibility of apparent diffusion coefficients with breath-hold and respiratory-triggered diffusion-weighted MR imaging. Radiology 255:815–823
American College of Radiology (2017) Liver imaging reporting and data system. American College of Radiology. https://www.acr.org/Quality-Safety/Resources/LIRADS. Accessed 1 Jul 2017
Kundel HL, Polansky M (2003) Measurement of observer agreement. Radiology 228:303–308
Nougaret S, Vargas HA, Lakhman Y et al (2016) Intravoxel incoherent motion-derived histogram metrics for assessment of response after combined chemotherapy and radiation therapy in rectal cancer: initial experience and comparison between single-section and volumetric analyses. Radiology 280:446–454
Kitao A, Matsui O, Yoneda N et al (2012) Hypervascular hepatocellular carcinoma: correlation between biologic features and signal intensity on gadoxetic acid-enhanced MR images. Radiology 265:780–789
de Martel C, Maucort-Boulch D, Plummer M, Franceschi S (2015) World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology 62:1190–1200
Kao WY, Su CW, Chau GY, Lui WY, Wu CW, Wu JC (2011) A comparison of prognosis between patients with hepatitis B and C virus-related hepatocellular carcinoma undergoing resection surgery. World J Surg 35:858–867
Zhou Y, Si X, Wu L, Su X, Li B, Zhang Z (2011) Influence of viral hepatitis status on prognosis in patients undergoing hepatic resection for hepatocellular carcinoma: a meta-analysis of observational studies. World J Surg Oncol 9:108
Jonas S, Bechstein WO, Steinmüller T et al (2001) Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology 33:1080–1086
Kim SU, Jung KS, Lee S et al (2014) Histological subclassification of cirrhosis can predict recurrence after curative resection of hepatocellular carcinoma. Liver Int 34:1008–1017
Granata V, Fusco R, Catalano O et al (2016) Intravoxel incoherent motion (IVIM) in diffusion-weighted imaging (DWI) for hepatocellular carcinoma: correlation with histologic grade. Oncotarget 7:79357–79364
Nakanishi M, Chuma M, Hige S et al (2012) Relationship between diffusion-weighted magnetic resonance imaging and histological tumor grading of hepatocellular carcinoma. Ann Surg Oncol 19:1302–1309
Renzulli M, Brocchi S, Cucchetti A et al (2016) Can current preoperative imaging be used to detect microvascular invasion of hepatocellular carcinoma? Radiology 279:432–442
Colecchia A, Schiumerini R, Cucchetti A et al (2014) Prognostic factors for hepatocellular carcinoma recurrence. World J Gastroenterol 20:5935–5950
Jerome NP, d’Arcy JA, Feiweier T et al (2016) Extended T2-IVIM model for correction of TE dependence of pseudo-diffusion volume fraction in clinical diffusion-weighted magnetic resonance imaging. Phys Med Biol 61:N667–N680
Lemke A, Laun FB, Simon D, Stieltjes B, Schad LR (2010) An in vivo verification of the intravoxel incoherent motion effect in diffusion-weighted imaging of the abdomen. Magn Reson Med 64:1580–1585
Andreou A, Koh DM, Collins DJ et al (2013) Measurement reproducibility of perfusion fraction and pseudodiffusion coefficient derived by intravoxel incoherent motion diffusion-weighted MR imaging in normal liver and metastases. Eur Radiol 23:428–434
Jerome NP, Miyazaki K, Collins DJ et al (2017) Repeatability of derived parameters from histograms following non-Gaussian diffusion modelling of diffusion-weighted imaging in a paediatric oncological cohort. Eur Radiol 27:345–353
Cohen AD, Schieke MC, Hohenwalter MD, Schmainda KM (2015) The effect of low b-values on the intravoxel incoherent motion derived pseudodiffusion parameter in liver. Magn Reson Med 73:306–311
Dyvorne H, Jajamovich G, Kakite S, Kuehn B, Taouli B (2014) Intravoxel incoherent motion diffusion imaging of the liver: optimal b-value subsampling and impact on parameter precision and reproducibility. Eur J Radiol 83:2109–2113
Kakite S, Dyvorne H, Besa C et al (2015) Hepatocellular carcinoma: short-term reproducibility of apparent diffusion coefficient and intravoxel incoherent motion parameters at 3.0T. J Magn Reson Imaging 41:149–156
Xia Y, Yan ZL, Xi T et al (2012) A case-control study of correlation between preoperative serum AFP and recurrence of hepatocellular carcinoma after curative hepatectomy. Hepatogastroenterology 59:2248–2254
Ma WJ, Wang HY, Teng LS (2013) Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J Surg Oncol 11:212
Cho SJ, Yoon JH, Hwang SS, Lee HS (2007) Do young hepatocellular carcinoma patients with relatively good liver function have poorer outcomes than elderly patients? J Gastroenterol Hepatol 22:1226–1231
Wai CT, Woon WA, Tan YM, Lee KH, Tan KC (2012) Younger age and presence of macrovascular invasion were independent significant factors associated with poor disease-free survival in hepatocellular carcinoma patients undergoing living donor liver transplantation. Transplant Proc 44:516–519
Funding
The authors state that this study has received funding by National Natural Science Foundation of China grant 81271562 (JW) and Science and Technology Program of Guangzhou, China 201704020016 (JW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jin Wang.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Kuang, S., Shan, Q. et al. Can IVIM help predict HCC recurrence after hepatectomy?. Eur Radiol 29, 5791–5803 (2019). https://doi.org/10.1007/s00330-019-06180-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06180-1