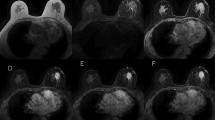
Abstract
Objectives
To evaluate the diagnostic utility of electric properties tomography (EPT) in differentiating benign from malignant breast lesions in comparison with dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI).
Methods
In this institutional review board-approved retrospective study, 116 consecutive patients with 141 breast lesions (50 benign and 91 malignant) underwent 3-T MRI, including 3D turbo-spin echo (TSE) sequence and standard DCE-MRI scans between January 2014 and January 2017. The lesions were segmented semi-automatically using subtraction DCE-MR images, and they were registered to the phase images from 3D TSE. The mean conductivity of the lesion was obtained from phase-based reconstruction of lesions. From the DCE-MRI, initial enhancement rate (IER) and signal enhancement ratio (SER) were calculated from signal intensity (SI) as follows: IER = (SIearly - SIpre)/SIpre, SER = (SIearly - SIpre)/(SIdelayed - SIpre). The parameters from EPT and the DCE-MRI were compared between benign and malignant lesions.
Results
There was significant difference in mean conductivity (0.14 ± 1.77 vs 1.14 ± 1.36 S/m, p < 0.0001) and SER (0.77 ± 0.28 vs 1.04 ± 0.25, p < 0.0001) between benign and malignant lesions, but not in IER (p = 0.06). Receiver operating curve (ROC) analysis revealed that the area under the curve (AUC) of the mean conductivity and SER was 0.71 and 0.80, respectively, without significant difference (p = 0.15).
Conclusions
The mean conductivity of EPT was significantly different between benign and malignant breast lesions as well as kinetic parameter or SER from DCE-MRI.
Key Points
• The conductivity of malignant lesions was higher than that of benign lesions.
• EPT helps differentiatie benign from malignant lesions.
• Diagnostic ability of EPT was not significantly different from that of DCE-MRI.





Similar content being viewed by others
Abbreviations
- DCE-MRI:
-
Dynamic contrast-enhanced magnetic resonance imaging
- EPT:
-
Electric properties tomography
- FGT:
-
Fibrograndular tissue
- IER:
-
Initial enhancement rate
- SER:
-
Signal enhancement ratio
- T2-VISTA:
-
T2-volume isotropic turbo spin echo acquisition
- TSC:
-
Tissue sodium concentration
References
Kriege M, Brekelmans CT, Boetes C et al (2004) Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 351:427–437
Warner E, Messersmith H, Causer P, Eisen A, Shumak R, Plewes D (2008) Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med 148:671–679
Kuhl CK, Schrading S, Leutner CC et al (2005) Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol 23:8469–8476
Warner E, Plewes DB, Hill KA et al (2004) Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA 292:1317–1325
Lehman CD, Blume JD, Weatherall P et al (2005) Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer 103:1898–1905. https://doi.org/10.1002/cncr.20971
American College of Radiology (2013) Breast imaging reporting and data system (BI-RADS), 5th edn. American College of Radiology, Reston
Brasch RC, Weinmann H-J, Wesbey GE (1984) Contrast-enhanced NMR imaging: animal studies using gadolinium-DTPA complex. AJR 142:625–630
Revel D, Brasch RC, Paajanen H et al (1986) Gd-DTPA contrast enhancement and tissue differentiation in MR imaging of experimental breast carcinoma. Radiology 158:319–323
Heuser LS, Miller FN (1986) Differential macromolecular leakage from the vasculature of tumors. Cancer 57:461–464
Wikström MG, Moseley ME, White DL et al (1989) Contrast-enhanced MRI of tumors. Comparison of Gd-DTPA and a macromolecular agent. Invest Radiol 24:609–615
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2013) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782
Katscher U, Kim DH, Seo JK (2013) Recent progress and future challenges in MR electric properties tomography. Comput Math Methods Med 2013:1–11
Ouwerkerk R, Jacobs MA, Macura KJ et al (2007) Elevated tissue sodium concentration in malignant breast lesions detected with non-invasive 23Na MRI. Breast Cancer Res Treat 106:151–160
Cameron IL, Smith NK, Pool TB, Sparks RL (1980) Intracellular concentration of sodium and other elements as related to mitogenesis and oncogenesis in vivo. Cancer Res 40:1493–1500
van Lier A, de Bruin P, Aussenhofer S et al (2013) 23Na-MRI and EPT: are sodium concentration and electrical conductivity at 298 MHz (7 T) related? Proc Intl Soc Mag Reson Med 21, Salt Palace Convention Center, Salt Lake City
Lagarde AE, Pouysségur JM (1986) The Na+:H+ antiport in cancer. Cancer Biochem Biophys 9:1–14
Ouwerkerk R, Bleich KB, Gillen JS, Pomper MG, Bottomley PA et al (2003) Tissue sodium concentration in human brain tumors as measured with 23Na MR imaging. Radiology 227:529–537
Huhndorf M, Stehning C, Rohr A, Helle M, Katscher U, Jansen O (2013) Systematic brain tumor conductivity study with optimized EPT Sequence and reconstruction algorithm. Proc 21st Intl Soc Mag Reson Med, Salt Palace Convention Center, Salt Lake City, 20-26 April 2013
Voigt T, Väterlein O, Stehning C, Katscher U, Fiehler J (2011) In vivo glioma characterization using MR conductivity imaging. Proc Int Soc Magn Reson Med 19, Palais des congrès de Montréal, Montréal
van Lier AL, Hoogduin JM, Polders DL (2011) Electrical conductivity imaging of brain tumours. Proc 19th Sci Meet Int Soc Magn Reson Med, Palais des congrès de Montréal, Montréal, 7-13 May 2011
Tha KK, Katscher U, Yamaguchi S et al (2018) Noninvasive electrical conductivity measurement by MRI: a test of its validity and the electrical conductivity characteristics of glioma. Eur Radiol 28:348–355
Shin J, Kim MJ, Lee J et al (2015) Initial study on in vivo conductivity mapping of breast cancer using MRI: in vivo conductivity mapping of breast cancer. J Magn Reson Imaging 42:371–378
Kim SY, Shin J, Kim DH et al (2016) Correlation between conductivity and prognostic factors in invasive breast cancer using magnetic resonance electric properties tomography (MREPT). Eur Radiol 26:2317–2326
Abe H, Mori N, Tsuchiya K et al (2016) Kinetic analysis of benign and malignant breast lesions with ultrafast dynamic contrast-enhanced MRI: comparison with standard kinetic assessment. AJR Am J Roentgenol 207:1159–1166
Katscher U, Voigt T, Findeklee C, Vernickel P, Nehrke K, Dössel O (2009) Determination of electric conductivity and local SAR via B1 mapping. IEEE Trans Med Imaging 28:1365–1374
Sujji GE, Lakshmi YVS, Jiji GW (2013) MRI brain image segmentation based on thresholding. Int J Adv Comput Res 3:97–101
Katscher U, van den Berg CAT (2017) Electric properties tomography: biochemical, physical and technical background, evaluation and clinical applications. NMR Biomed 30:e3729. https://doi.org/10.1002/nbm.3729
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Zaric O, Pinker K, Zbyn S et al (2016) Quantitative sodium MR imaging at 7 T: initial results and comparison with diffusion-weighted imaging in patients with breast tumors. Radiology 280:39–48
Zou Y, Guo Z (2003) A review of electrical impedance techniques for breast cancer detection. Med Eng Phys 25:79–90
Poplack SP, Tosteson TD, Wells WA et al (2007) Electromagnetic breast imaging: results of a pilot study in women with abnormal mammograms. Radiology 243:350–359
Joines WT, Zhang Y, Li C, Jirtle RL (1994) The measured electrical properties of normal and malignant human tissues from 50 to 900 MHz. Med Phys 21:547–550
Surowiec AJ, Stuchly SS, Barr JR, Swarup A (1988) Dielectric properties of breast carcinoma and the surrounding tissues. IEEE Trans Biomed Eng 35:257–263
Haacke E, Petropoulos LS, Nilges EW, Wu D (1991) Extraction of conductivity and permittivity using MRI. Phys Med Biol 38:723–741
Lee J, Choi N, Seo JK, Kim DH (2017) Magnetic resonance electrical properties tomography for small anomalies using boundary conditions: a simulation study. Med Phys 44:4773–4785
Kim SY, Shin J, Kim DH et al (2018) Correlation between electrical conductivity and apparent diffusion coefficient in breast cancer: effect of necrosis on magnetic resonance imaging. Eur Radiol 28:3204–3214
Acknowledgements
This research was partly supported by Philips Healthcare. The authors thank Sharon Harris in the University of Chicago, for her kind support. The authors thank Yumi Fujimoto, Shomo Chou in Tohoku University for their kind support.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Hiroyuki Abe.
Conflict of interest
The authors (Naoko Mori, Keiko Tsuchiya, Deepa Sheth, Shunji Mugikura, Kei Takase and Hiroyuki Abe) of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Ulrich Katscher is an employee of Philips Technologie GmbH, Research Laboratories.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional review board approval was obtained.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Performed at one institution
Electronic supplementary material
ESM 1
(DOCX 89 kb)
Rights and permissions
About this article
Cite this article
Mori, N., Tsuchiya, K., Sheth, D. et al. Diagnostic value of electric properties tomography (EPT) for differentiating benign from malignant breast lesions: comparison with standard dynamic contrast-enhanced MRI. Eur Radiol 29, 1778–1786 (2019). https://doi.org/10.1007/s00330-018-5708-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5708-4