Abstract
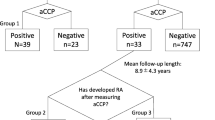
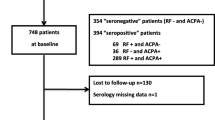
A family history of rheumatoid arthritis (RA) is a strong risk factor for developing RA, affecting both genetically and environmentally. However, whether family history provides clinically relevant information in the modern classification and treatment remains largely unknown. This study aimed to determine whether a family history of RA is associated with a different clinical presentation of RA and treatment response. We retrospectively evaluated the demographic data and disease activity of newly diagnosed RA patients at baseline, 1 year, and 2 years after onset, using the ANSWER (Kansai consortium for the well-being of rheumatic disease patients) cohort data. Thirty-one patients (11.9%) among 260 newly diagnosed RA patients had a family history of RA up to second degree. There was no significant difference in the age at onset, time from onset to first visit, sex, positivity or value of rheumatoid factor or anti-cyclic citrullinated peptide antibody (ACPA), or disease activity between patients with and without a family history of RA. However, patients who had a family history of RA and were ACPA positive showed significantly lower erythrocyte sedimentation rate, and C-reactive protein. Disease activity in patients with a family history was not worse at baseline, after 1 year or 2 years of treatment. The Larsen score 2 years after onset was equivalent between the patients with and without a family history of RA in ACPA-positive patients. Family history of RA in ACPA-positive patients is not associated with high disease activity at baseline and is not a predictor of poor outcome 2 years after onset.


Similar content being viewed by others
References
Smolen JS, Aletaha D, McInnes IB (2016) Rheumatoid arthritis. Lancet 388:2023–2038
Okada Y, Wu D, Trynka G et al (2014) Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 506:376–381
Frisell T, Saevarsdottir S, Askling J (2016) Family history of rheumatoid arthritis: an old concept with new developments. Nat Rev Rheumatol 12:335–343
Frisell T, Holmqvist M, Källberg H et al (2013) Familial risks and heritability of rheumatoid arthritis: role of rheumatoid factor/anti-citrullinated protein antibody status, number and type of affected relatives, sex, and age. Arthritis Rheum 65:2773–2782
Hensvold AH, Magnusson PKE, Joshua V et al (2015) Environmental and genetic factors in the development of anticitrullinated protein antibodies (ACPAs) and ACPA-positive rheumatoid arthritis: an epidemiological investigation in twins. Ann Rheum Dis 74:375–380
Van Der Woude D, Houwing-Duistermaat JJ, Toes REM et al (2009) Quantitative heritability of anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis. Arthritis Rheum 60:916–923
Eyre S, Bowes J, Diogo D et al (2012) High density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet 44:1336–1340
Hemminki K, Li X, Sundquist J, Sundquist K (2009) Familial associations of rheumatoid arthritis with autoimmune diseases and related conditions. Arthritis Rheum 60:661–668
Somers EC, Antonsen S, Pedersen L, Sørensen HT (2013) Parental history of lupus and rheumatoid arthritis and risk in offspring in a nationwide cohort study: does sex matter? Ann Rheum Dis 72:525
Grant SFA, Thorleifsson G, Frigge ML et al (2001) The inheritance of rheumatoid arthritis in Iceland. Arthritis Rheum 44:2247–2254
Ling SF, Viatte S, Lunt M et al (2016) HLA–DRB1 amino acid positions 11/13, 71, and 74 are associated with inflammation level, disease activity, and the health assessment questionnaire score in patients with inflammatory polyarthritis. Arthritis Rheumatol 68:2618–2628
Viatte S, Plant D, Han B et al (2015) Association of HLA-DRB1 haplotypes with rheumatoid arthritis severity, mortality, and treatment response. JAMA J Am Med Assoc 313:1645–1656
Knevel R, Klein K, Somers K et al (2014) Identification of a genetic variant for joint damage progression in autoantibody-positive rheumatoid arthritis. Ann Rheum Dis 73:2038–2046
de Rooy DPC, Zhernakova A, Tsonaka R et al (2014) A genetic variant in the region of MMP-9 is associated with serum levels and progression of joint damage in rheumatoid arthritis. Ann Rheum Dis 73:1163–1169
Viatte S, Barton A (2017) Genetics of rheumatoid arthritis susceptibility, severity, and treatment response. Semin Immunopathol 38:395–408
Aletaha D, Neogi T, Silman AJ (2010) 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Ann Rheum Dis 69:1580–1588
Smolen J, Landewé R, Breedveld F et al (2014) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 73:492–509
Frisell T, Saevarsdottir S, Askling J (2016) Does a family history of RA influence the clinical presentation and treatment response in RA? Ann Rheum Dis 75:1120–1125
Ebina K, Hashimoto M, Yamamoto W et al (2019) Drug tolerability and reasons for discontinuation of seven biologics in elderly patients with rheumatoid arthritis—the ANSWER cohort study. PLoS One 14:e0216624
Hashimoto M, Furu M, Yamamoto W et al (2018) Factors associated with the achievement of biological disease-modifying antirheumatic drug-free remission in rheumatoid arthritis: the ANSWER cohort study. Arthritis Res Ther 20:165
Ebina K, Hashimoto M, Yamamoto W et al (2019) Drug tolerability and reasons for discontinuation of seven biologics in 4466 treatment courses of rheumatoid arthritis—the ANSWER cohort study. Arthritis Res Ther 21:91
Ebina K, Hashimoto M, Yamamoto W et al (2018) Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis—the ANSWER cohort study. PLoS One 13:e0194130
Arnett FC, Edworthy SM, Bloch DA et al (1988) The american rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Aletaha D, Neogi T, Silman AJ et al (2010) 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum 62:2569–2581
Larsen A (1995) How to apply Larsen score in evaluating radiographs of rheumatoid arthritis in longterm studies? J Rheumatol 22:1974–1975
Murata K, Ito H, Hashimoto M et al (2018) Elderly onset of early rheumatoid arthritis is a risk factor for bone erosions, refractory to treatment: KURAMA cohort. Int J Rheum Dis 22:1084–1093
Jawaheer D, Lum RF, Amos CI et al (2004) Clustering of disease features within 512 multicase rheumatoid arthritis families. Arthritis Rheum 50:736–741
Umicevic Mirkov M, Cui J, Vermeulen SH et al (2013) Genome-wide association analysis of anti-TNF drug response in patients with rheumatoid arthritis. Ann Rheum Dis 72:1375–1381
Radstake TR, Barrera P, Albers MJ et al (2001) Genetic anticipation in rheumatoid arthritis in Europe. European Consortium on Rheumatoid Arthritis Families. J Rheumatol 28:962–967
Radstake TRDJ, Barrera P, Albers JMC et al (2000) Familial vs sporadic rheumatoid arthritis (RA). A prospective study in an early RA inception cohort. Rheumatology 39:267–273
Terao C, Ohmura K, Kochi Y et al (2015) Anti-citrullinated peptide/protein antibody (ACPA)-negative RA shares a large proportion of susceptibility loci with ACPA-positive RA: a meta-analysis of genome-wide association study in a Japanese population. Arthritis Res Ther 17:104
Han B, Diogo D, Eyre S et al (2014) Fine mapping seronegative and seropositive rheumatoid arthritis to shared and distinct HLA alleles by adjusting for the effects of heterogeneity. Am J Hum Genet 94:522–532
Padyukov L, Seielstad M, Ong RTH et al (2011) A genome-wide association study suggests contrasting associations in ACPA-positive versus ACPA-negative rheumatoid arthritis. Ann Rheum Dis 70:259–265
Plant D, Wilson AG, Barton A (2014) Genetic and epigenetic predictors of responsiveness to treatment in RA. Nat Rev Rheumatol 10:329–337
Acknowledgements
We wish to thank all medical staff at all institutions participating in the ANSWER cohort for providing the data. This manuscript was proofread by Enago, Academic Proofreading Service.
Funding
The study reported in this publication uses ANSWER Cohort supported by grants from five pharmaceutical companies (Chugai Pharmaceutical Co. Ltd, Eisai Co., Ltd., Janssen Pharmaceutical K.K., Ono Pharmaceutical Co., Ltd. and Sanofi S.A.) and an information technology services company (CAC). Department of Advanced Medicine for Rheumatic Diseases, Kyoto University Graduate School of Medicine is supported by Nagahama City, Shiga, Japan and four pharmaceutical companies (Mitsubishi Tanabe Pharma Corporation, Chugai Pharmaceutical Co. Ltd, UCB Japan Co. Ltd, and AYUMI Pharmaceutical Co.). KURAMA cohort study, one of cohorts consisting ANSWER cohort, is supported by grant from Daiichi Sankyo Co. Ltd. KN and TT belongs to the department which is financially supported by four pharmaceutical companies (Mitsubishi Tanabe Pharma Corporation, Chugai Pharmaceutical Co., Asahi Kasei Pharma Corporation, Eisai Co., Takeda Pharmaceutical Company Limited). This study is conducted as investigator-initiated study. These companies had no role in the design of the study, the collection or analysis of the data, the writing of the manuscript or decision to submit the manuscript for the publication.
Author information
Authors and Affiliations
Contributions
KM conceived and designed the study. KM, MH, WS, HA, KN, TT, MK, YM KE, RH, SJ, AO, KM, MT, HI, TM, and SM recruited the patients and collected the data. KM and WY analyzed the data. KM prepared the initial draft of the manuscript. All the authors were involved in revising the manuscript critically for content. All authors read and approved the final manuscript. They also agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
KMurat received a speaking fee and/or consulting fee from Eisai Co., Ltd. MH received a research grant and/or speaker fee from Astellas Pharma Inc., Mitsubishi Tanabe Pharma Corporation, and Bristol-Myers Squibb. TT is affiliated with a department that is financially supported by six pharmaceutical companies (Mitsubishi Tanabe Pharma Corporation, Chugai Pharmaceutical Co. Ltd, AYUMI Pharmaceutical Co., Astellas Pharma, Eisai Co., Ltd., and Takeda Pharmaceutical Company Limited). KN received a research grant and/or speaker fee from Mitsubishi Tanabe Pharma Corporation, Chugai Pharmaceutical Co., Bristol-Myers Squibb, Eli Lily, Pfizer Inc, Janssen Pharmaceutical K.K., UCB Japan Co. Ltd, and Asahi Kasei Pharma Corporation. TT received a research grant from Chugai Pharmaceutical Co. Ltd, and a speaker fee from Astellas Pharma Inc., Chugai Pharmaceutical Co. Ltd, Mitsubishi Tanabe Pharma Corporation, Bristol-Myers Squibb, Eli Lily, Pfizer Inc, Janssen Pharmaceutical K.K., UCB Japan Co. Ltd, Astellas Pharma, and Asahi Kasei Pharma Corporation. YM received a research grant and/or speaker fee from Eli Lily, Chugai Pharmaceutical Co. Ltd., Pfizer Inc, Bristol-Myers Squibb and Mitsubishi Tanabe Pharma Corporation. KE received a research grant and/or speaker fee from Asahi Kasei Corporation, Astellas Pharma Inc., Chugai Pharmaceutical Co. Ltd, Daiichi Sankyo Co. Ltd., Eisai Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Ono Pharmaceutical Co., Ltd., Taisho-toyama, and UCB Japan Co. Ltd. SJ received a research grant and/or speaker fee from Mitsubishi Tanabe Pharma Corporation, Chugai Pharmaceutical Co., Asahi Kasei Pharma Corporation, Eli Lilly and Company, and AbbVie GK. KMurak has received speaking fees, and/or consulting fees from Eisai Co. Ltd, Chugai Pharmaceutical Co. Ltd., Pfizer Inc., Bristol-Myers Squibb, Mitsubishi Tanabe Pharma Corporation, UCB Japan Co. Ltd, Daiichi Sankyo Co. Ltd. and Astellas Pharma Inc. M.T. has received research grants and/or speaker fees from AbbVie GK, Asahi Kasei Pharma Corporation, Astellas Pharma Inc., Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Eli Lilly and Company, Pfizer Inc., UCB Japan Co., Ltd., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, Novartis Pharma K.K., Taisho Pharma Co., Ltd, and Takeda Pharmaceutical Company Limited,. H.I. has received a research grant and/or speaker fee from Bristol-Myers Squibb, Astellas Pharma Inc., Asahi Kasei Pharma Corporation, and Kyocera Medical Co. TM has received research grants and/or speakers’ fees from Astellas Pharma Inc., Asahi Kasei Corporation, AYUMI Pharmaceutical Co., Chugai Pharmaceutical Co. Ltd, Daiichi Sankyo Co. Ltd., Eisai Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Pfizer Inc., and Sanofi S.A. SM has received a research grant and/or speaker fee from Eisai Co., Ltd., Chugai Pharmaceutical Co. Ltd, and Ono Pharmaceutical Co. All other authors have declared no conflicts of interests.
Ethics approval
This observational study was conducted as per the Declaration of Helsinki and was approved by the ethics committees of all seven institutes: Kyoto University (2016-03-24/approved number R053), Osaka University (2015-11-04/approved number 15300), Osaka Medical College (2014-07-14/approved number 1529), Kansai Medical University (2017-11-21/approved number 2014625), Kobe University (2015-03-20/approved number 1738), Nara Medial University (2018-01-23/approved number 1692), and Osaka Red Cross Hospital (2015-09-01/approved number 644).
Informed consent
The board of Osaka University Hospital Ethical Committee waived the requirement for patients’ informed consent because of the anonymous nature of the data. Written informed consent was obtained from the participants in other institutes.
Plagiarism
No parts of the manuscript were copied from elsewhere.
Prior conference presentation
Part of the data was presented at the 63rd annual general assembly and scientific meeting of the Japan College of Rheumatology and the 5th annual meeting of Japanese Society of Osteoimmunogy.
Congress abstract
Koichi Murata, Motomu Hashimoto, Wataru Yamamoto, Yonsu Son, Hideki Amuro, Koji Nagai, Tohru Takeuchi, Masaki Katayama, Yuichi Maeda, Kosuke Ebina, Ryota Hara, Sadao Jinno, Akira Onishi, Kosaku Murakami, Masao Tanaka, Hiromu Ito, Tsuneyo Mimori and Shuichi Matsuda. Does a family history of RA influence the clinical presentation and treatment response in RA? ANSWER Cohort—[abstract] In: the 63rd annual general assembly and scientific meeting of the Japan College of Rheumatology; 2019 Apr 15–14; Kyoto, Kyoto, Japan. Abstract nr P3-007. Koichi Murata, Motomu Hashimoto, Wataru Yamamoto, Yonsu Son, Hideki Amuro, Koji Nagai, Tohru Takeuchi, Masaki Katayama, Yuichi Maeda, Kosuke Ebina, Ryota Hara, Sadao Jinno, Akira Onishi, Kosaku Murakami, Masao Tanaka, Hiromu Ito, Tsuneyo Mimori and Shuichi Matsuda. Does a family history of RA influence the clinical presentation and treatment response in RA? ANSWER Cohort—[abstract] In: the 5th annual meeting of Japanese Society of Osteoimmunogy; 2019 June 25–27; Ishigaki, Okinawa, Japan. Abstract nr P2-1.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Murata, K., Hashimoto, M., Yamamoto, W. et al. The family history of rheumatoid arthritis in anti-cyclic citrullinated peptide antibody-positive patient is not a predictor of poor clinical presentation and treatment response with modern classification criteria and treatment strategy: the ANSWER cohort study. Rheumatol Int 40, 217–225 (2020). https://doi.org/10.1007/s00296-019-04464-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-019-04464-9