Abstract
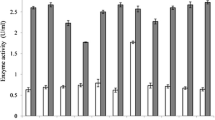
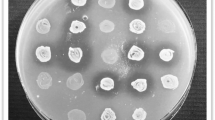
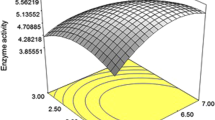
Phytate-linked nutritional deficiency disorders have plagued poultry for centuries. The application of exogenous phytases in poultry feed has served as a solution to this problem. However, they are linked to certain limitations which include thermal instability during prolonged feed processing. Therefore, in this study, Streptococcus thermophilus 2412 based phytase stability was assessed at higher temperatures up to 90 °C. This was followed by probiotic validation of the same bacterium in an in vitro intestinal model. Bacterial phytase showed thermostability up to 70 °C with a recorded activity of 9.90 U. The bacterium was viable in the intestinal lumen as indicated by the cell count of 6.10 log(CFU/mL) after 16 h. It also showed acid tolerance with a stable cell count of 5.01 log(CFU/mL) after 16 h of incubation at pH 2. The bacterium displayed bile tolerance yielding a cell count of 6.36 log(CFU/mL) in the presence of 0.3% bile. Bacterial susceptibility was observed toward all tested antibiotics with a maximum zone of 20 mm against clindamycin. The maximum antagonistic activity was observed against Staphylococcus aureus, Serratia marcescens, and Escherichia coli with inhibition zone diameters up to 10 mm. The above characteristics prove that S. thermophilus 2412 can be used as an effective phytase-producing poultry probiotic.





Similar content being viewed by others
References
Park YH, Hamidon F, Rajangan C et al (2016) Application of probiotics for the production of safe and high-quality poultry meat. Korean J Food Sci Anim Resour 36:567–576. https://doi.org/10.5851/kosfa.2016.36.5.567
Widodo AE, Nolan JV, Akter M et al (2018) Response of broiler chickens to triticale-based diets supplemented with microbial enzymes (1. Growth and intestinal function). Poult Sci J 6:25–40. https://doi.org/10.22069/psj.2018.13811.1280
Fujimoto H, Fujita N, Takada R (2018) Effects of a rice diet and phytase addition on growth performance, tissue weights, phosphorus and nitrogen retention, and on liver threonine dehydrogenase, malic enzyme and fatty acid synthase activities in broiler chicks. Anim Sci J 89:770–776. https://doi.org/10.1111/asj.12991
Campasino A, York T, Wyatt C et al (2014) Effect of increasing supplemental phytase concentration in diets fed to Hubbard × Cobb 500 male broilers from 1 to 42 days of age1. J Appl Poult Res 23:705–714. https://doi.org/10.3382/japr.2014-00999
Muszyński S, Tomaszewska E, Kwiecień M et al (2018) Effect of dietary phytase supplementation on bone and hyaline cartilage development of broilers fed with organically complexed copper in a Cu-deficient diet. Biol Trace Elem Res 182:339–353. https://doi.org/10.1007/s12011-017-1092-1
Ahmadi H, Rodehutscord M (2012) A meta-analysis of responses to dietary nonphytate phosphorus and phytase in laying hens. Poult Sci 91:2072–2078. https://doi.org/10.3382/ps.2012-02193
Bradbury EJ, Wilkinson SJ, Cronin GM et al (2018) Effects of phytase, calcium source, calcium concentration and particle size on broiler performance, nutrient digestibility and skeletal integrity. Anim Prod Sci 58:271–283. https://doi.org/10.1071/AN16175
Dos Santos TT, Srinongkote S, Bedford MR, Walk CL (2013) Effect of high phytase inclusion rates on performance of broilers fed diets not severely limited in available phosphorus. Asian-Australas J Anim Sci 26:227–232. https://doi.org/10.5713/ajas.2012.12445
Singh PK (2008) Significance of phytic acid and supplemental phytase in chicken nutrition: a review. Worlds Poult Sci J 64:553–580. https://doi.org/10.1017/s0043933908000202
Selle PH, Ravindran V (2007) Microbial phytase in poultry nutrition. Anim Feed Sci Technol 135:1–41. https://doi.org/10.1016/j.anifeedsci.2006.06.010
Ravindran V (2013) Feed enzymes: The science, practice, and metabolic realities. J Appl Poult Res 22:628–636. https://doi.org/10.3382/japr.2013-00739
Abdel-Megeed A, Tahir A (2015) Reduction of phosphorus pollution from broilers waste through supplementation of wheat based broilers feed with phytase. J Chem. https://doi.org/10.1155/2015/867014
Dhama K, Tiwari R, Khan RU et al (2014) Growth promoters and novel feed additives improving poultry production and health, bioactive principles and beneficial applications: the trends and advances-a review. Int J Pharmacol 10:129–159. https://doi.org/10.3923/ijp.2014.129.159
Patterson JA, Burkholder KM (2003) Application of prebiotics and probiotics in poultry production. Poult Sci 82:627–631. https://doi.org/10.1093/ps/82.4.627
Kabir SML (2009) The role of probiotics in the poultry industry. Int J Mol Sci 10:3531–3546. https://doi.org/10.3390/ijms10083531
Askelson TE, Campasino A, Lee JT, Duong T (2014) Evaluation of phytate-degrading Lactobacillus culture administration to broiler chickens. Appl Environ Microbiol 80:943–950. https://doi.org/10.1128/AEM.03155-13
Aluwong T, Sumanu VO, Ayo JO et al (2017) Daily rhythms of cloacal temperature in broiler chickens of different age groups administered with zinc gluconate and probiotic during the hot-dry season. Physiol Rep 5:e13314. https://doi.org/10.14814/phy2.13314
Linares DM, O’Callaghan TF, O’Connor PM et al (2016) Streptococcus thermophilus APC151 strain is suitable for the manufacture of naturally gaba-enriched bioactive yogurt. Front Microbiol 7:1876. https://doi.org/10.3389/fmicb.2016.01876
Jäger R, Purpura M, Stone JD et al (2016) Probiotic Streptococcus thermophilus FP4 and Bifidobacterium breve BR03 supplementation attenuates performance and range-of-motion decrements following muscle damaging exercise. Nutrients 8:E642. https://doi.org/10.3390/nu8100642
Vitetta L, Llewellyn H, Oldfield D (2019) Gut dysbiosis and the intestinal microbiome: Streptococcus thermophilus a key probiotic for reducing uremia. Microorganisms 7:228. https://doi.org/10.3390/microorganisms7080228
Priyodip P, Balaji S (2018) Microbial degradation of myo-inositol hexakisphosphate (IP6): specificity, kinetics, and simulation. 3 Biotech 8:268. https://doi.org/10.1007/s13205-018-1302-3
Bae HD, Yanke LJ, Cheng KJ, Selinger LB (1999) A novel staining method for detecting phytase activity. J Microbiol Methods 39:17–22. https://doi.org/10.1016/S0167-7012(99)00096-2
Dokuzparmak E, Sirin Y, Cakmak U, Saglam Ertunga N (2017) Purification and characterization of a novel thermostable phytase from the thermophilic Geobacillus sp. TF16. Int J Food Prop 20:1104–1116. https://doi.org/10.1080/10942912.2016.1203930
Salas-Jara M, Ilabaca A, Vega M et al (2016) Biofilm forming Lactobacillus: New challenges for the development of probiotics. Microorganisms 4:35. https://doi.org/10.3390/microorganisms4030035
Christensen GD, Simpson WA, Bisno AL, Beachey EH (1982) Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun 37:318–326
Priyodip P, Balaji S (2019) A preliminary study on probiotic characteristics of Sporosarcina spp. for poultry applications. Curr Microbiol 76:448–461. https://doi.org/10.1007/s00284-019-01647-2
Canzi E, Guglielmetti S, Mora D et al (2005) Conditions affecting cell surface properties of human intestinal bifidobacteria. Antonie Van Leeuwenhoek 88:207–219. https://doi.org/10.1007/s10482-005-6501-3
Rosenberg M, Gutnick D, Rosenberg E (1980) Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett 9:29–33. https://doi.org/10.1111/j.1574-6968.1980.tb05599.x
Priyodip P, Balaji S (2019) An in vitro chicken gut model for the assessment of phytase producing bacteria. 3Biotech 9:294. https://doi.org/10.1007/s13205-019-1825-2
Alzawqari MH, Al-Baadani HH, Alhidary IB et al (2016) Effect of taurine and bile acid supplementation and their interaction on performance, serum components, ileal viscosity and carcass characteristics of broiler chickens. S Afr J Anim Sci 46:448–457. https://doi.org/10.4314/sajas.v46i4.13
Furtula V, Farrell EG, Diarrassouba F et al (2010) Veterinary pharmaceuticals and antibiotic resistance of Escherichia coli isolates in poultry litter from commercial farms and controlled feeding trials. Poult Sci 89:180–188. https://doi.org/10.3382/ps.2009-00198
Bauer AW, Perry DM, Kirby WMM (1959) Single-disk antibiotic-sensitivity testing of Staphylococci. AMA Arch Intern Med 104:208. https://doi.org/10.1001/archinte.1959.00270080034004
Yin Y, Lei F, Zhu L et al (2010) Exposure of different bacterial inocula to newborn chicken affects gut microbiota development and ileum gene expression. ISME J 4:367–376. https://doi.org/10.1038/ismej.2009.128
Farhat-Khemakhem A, Blibech M, Boukhris I et al (2018) Assessment of the potential of the multi-enzyme producer Bacillus amyloliquefaciens US573 as alternative feed additive. J Sci Food Agric 98:1208–1215. https://doi.org/10.1002/jsfa.8574
Rocky-Salimi K, Hashemi M, Safari M, Mousivand M (2016) A novel phytase characterized by thermostability and high pH tolerance from rice phyllosphere isolated Bacillus subtilis B.S.46. J Adv Res 7:381–390. https://doi.org/10.1016/j.jare.2016.02.003
Igbasan FA, Männer K, Miksch G et al (2000) Comparative studies on the in vitro properties of phytases from various microbial origins. Arch Tierernahr 53:353–373. https://doi.org/10.1080/17450390009381958
Borgi MA, Boudebbouze S, Aghajari N et al (2014) The attractive recombinant phytase from Bacillus licheniformis: biochemical and molecular characterization. Appl Microbiol Biotechnol 98:5937–5947. https://doi.org/10.1007/s00253-013-5421-9
Gulati HK, Chadha BS, Saini HS (2007) Production and characterization of thermostable alkaline phytase from Bacillus laevolacticus isolated from rhizosphere soil. J Ind Microbiol Biotechnol 34:91–98. https://doi.org/10.1007/s10295-006-0171-7
Vijayaraghavan P, Primiya RR, Prakash Vincent SG (2013) Thermostable alkaline phytase from Alcaligenes sp. in improving bioavailability of phosphorus in animal feed: in vitro analysis. ISRN Biotechnol. https://doi.org/10.5402/2013/394305
Kim Y-O, Kim H-K, Bae K-S et al (1998) Purification and properties of a thermostable phytase from Bacillus sp. DS11. Enzyme Microb Technol 22:2–7. https://doi.org/10.1016/S0141-0229(97)00096-3
Ha N-C, Oh B-C, Kim H-J et al (2000) Crystal structures of a novel, thermostable phytase in partially and fully calcium-loaded states. Nat Struct Biol 7:147–153. https://doi.org/10.1038/72421
Zhao T, Podtburg TC, Zhao P et al (2013) Reduction by competitive bacteria of Listeria monocytogenes in biofilms and Listeria bacteria in floor drains in a ready-to-eat poultry processing plant. J Food Prot 76:601–607. https://doi.org/10.4315/0362-028X.JFP-12-323
Lebeer S, Verhoeven TLA, Claes IJJ et al (2011) FISH analysis of Lactobacillus biofilms in the gastrointestinal tract of different hosts. Lett Appl Microbiol 52:220–226. https://doi.org/10.1111/j.1472-765X.2010.02994.x
Nallala V, Sadishkumar V, Jeevaratnam K (2017) Molecular characterization of antimicrobial Lactobacillus isolates and evaluation of their probiotic characteristics in vitro for use in poultry. Food Biotechnol 31:20–41. https://doi.org/10.1080/08905436.2016.1269289
Nguyen ATV, Nguyen DV, Tran MT et al (2015) Isolation and characterization of Bacillus subtilis CH16 strain from chicken gastrointestinal tracts for use as a feed supplement to promote weight gain in broilers. Lett Appl Microbiol 60:580–588. https://doi.org/10.1111/lam.12411
O’Toole G, Kaplan HB, Kolter R (2000) Biofilm formation as microbial development. Annu Rev Microbiol 54:49–79. https://doi.org/10.1146/annurev.micro.54.1.49
Yi K, Rasmussen AW, Gudlavalleti SK et al (2004) Biofilm formation by Neisseria meningitidis. Infect Immun 72:6132–6138. https://doi.org/10.1128/IAI.72.10.6132-6138.2004
Raghavendra P, Halami PM (2009) Screening, selection and characterization of phytic acid degrading lactic acid bacteria from chicken intestine. Int J Food Microbiol 133:129–134. https://doi.org/10.1016/j.ijfoodmicro.2009.05.006
Mota RM, Moreira JLS, Souza MR et al (2006) Genetic transformation of novel isolates of chicken Lactobacillus bearing probiotic features for expression of heterologous proteins: A tool to develop live oral vaccines. BMC Biotechnol 6:2. https://doi.org/10.1186/1472-6750-6-2
Gusils C, González SN, Oliver G (1999) Some probiotic properties of chicken lactobacilli. Can J Microbiol 45:981–987. https://doi.org/10.1139/w99-102
Pelletier C, Bouley C, Cayuela C et al (1997) Cell surface characteristics of Lactobacillus casei subsp. casei, Lactobacillus paracasei subsp. paracasei, and Lactobacillus rhamnosus strains. Appl Environ Microbiol 63:1725–1731
Gong X, Yu H, Chen J, Han B (2012) Cell surface properties of Lactobacillus salivarius under osmotic stress. Eur Food Res Technol 234:671–678. https://doi.org/10.1007/s00217-012-1677-z
Begovic J, Fira D, Terzic-Vidojevic A, Topisirovic L (2010) Influence of carbohydrates on cell properties of Lactobacillus rhamnosus. Cent Eur J Biol 5:103–110. https://doi.org/10.2478/s11535-009-0078-1
Sanchez-Ortiz AC, Luna-Gonzalez A, Campa-Cordova AI et al (2015) Isolation and characterization of potential probiotic bacteria from pustulose ark (Anadara tuberculosa) suitable for shrimp farming. Lat Am J Aquat Res 43:123–136. https://doi.org/10.3856/vol43-issue1-fulltext-11
Spivey MA, Dunn-Horrocks SL, Duong T (2014) Epithelial cell adhesion and gastrointestinal colonization of Lactobacillus in poultry. Poult Sci 93:2910–2919. https://doi.org/10.3382/ps.2014-04076
Kačániová M, Kmeť V, Čcuboň J (2006) Effect of Enterococcus faecium on the digestive tract of poultry as a probiotic. Turk J Vet Anim Sci 30:291–298
Beaussart A, El-Kirat-Chatel S, Sullan RMA et al (2014) Quantifying the forces guiding microbial cell adhesion using single-cell force spectroscopy. Nat Protoc 9:1049–1055. https://doi.org/10.1038/nprot.2014.066
Rokana N, Singh BP, Thakur N et al (2018) Screening of cell surface properties of potential probiotic lactobacilli isolated from human milk. J Dairy Res 85:347–354. https://doi.org/10.1017/S0022029918000432
Neeser JR, Granato D, Rouvet M et al (2000) Lactobacillus johnsonii La1 shares carbohydrate-binding specificities with several enteropathogenic bacteria. Glycobiology 10:1193–1199. https://doi.org/10.1093/glycob/10.11.1193
Turpin W, Humblot C, Noordine M-L et al (2012) Lactobacillaceae and cell adhesion: genomic and functional screening. PLoS ONE 7:e38034. https://doi.org/10.1371/journal.pone.0038034
Lukic J, Strahinic I, Milenkovic M et al (2014) Aggregation factor as an inhibitor of bacterial binding to gut mucosa. Microb Ecol 68:633–644. https://doi.org/10.1007/s00248-014-0426-1
Rajoka MSR, Hayat HF, Sarwar S et al (2018) Isolation and evaluation of probiotic potential of lactic acid bacteria isolated from poultry intestine. Microbiology 87:116–126. https://doi.org/10.1134/S0026261718010150
Ehrmann MA, Kurzak P, Bauer J, Vogel RF (2002) Characterization of lactobacilli towards their use as probiotic adjuncts in poultry. J Appl Microbiol 92:966–975. https://doi.org/10.1046/j.1365-2672.2002.01608.x
Corcoran BM, Stanton C, Fitzgerald GF, Ross RP (2005) Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl Environ Microbiol 71:3060–3067. https://doi.org/10.1128/AEM.71.6.3060-3067.2005
Hamon E, Marchioni E, Ennahar S et al (2013) Investigation of potential markers of acid resistance in Lactobacillus plantarum by comparative proteomics. J Appl Microbiol 116:134–144. https://doi.org/10.1111/jam.12339
Charalampopoulos D, Pandiella SS, Webb C (2003) Evaluation of the effect of malt, wheat and barley extracts on the viability of potentially probiotic lactic acid bacteria under acidic conditions. Int J Food Microbiol 82:133–141. https://doi.org/10.1016/S0168-1605(02)00248-9
Shokryazdan P, Kalavathy R, Sieo CC et al (2014) Isolation and characterization of Lactobacillus strains as potential probiotics for chickens. Pertanika J Trop Agric Sci 37:141–157
Kobierecka PA, Wyszyńska AK, Aleksandrzak-Piekarczyk T et al (2017) In vitro characteristics of Lactobacillus spp. strains isolated from the chicken digestive tract and their role in the inhibition of Campylobacter colonization. Microbiologyopen 6:e00512. https://doi.org/10.1002/mbo3.512
Yang Y, Latorre JD, Khatri B et al (2018) Characterization and evaluation of lactic acid bacteria candidates for intestinal epithelial permeability and Salmonella typhimurium colonization in neonatal turkey poults. Poult Sci 97:515–521. https://doi.org/10.3382/ps/pex311
Sahadeva RPK, Leong SF, Chua KH et al (2011) Survival of commercial probiotic strains to pH and bile. Int Food Res J 18:1515–1522
Ruiz L, Margolles A, Sánchez B (2013) Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front Microbiol 4:396. https://doi.org/10.3389/FMICB.2013.00396
Bhardwaj A, Gupta H, Kapila S et al (2010) Safety assessment and evaluation of probiotic potential of bacteriocinogenic Enterococcus faecium KH 24 strain under in vitro and in vivo conditions. Int J Food Microbiol 141:156–164. https://doi.org/10.1016/J.IJFOODMICRO.2010.05.001
Zommiti M, Connil N, Ben HJ, Ferchichi M (2017) Probiotic characteristics of Lactobacillus curvatus DN317, a strain isolated from chicken ceca. Probiotics Antimicrob Proteins 9:415–424. https://doi.org/10.1007/s12602-017-9301-y
Silhavy TJ, Kahne D, Walker S (2010) The bacterial cell envelope. Cold Spring Harb Perspect Biol 2:a000414. https://doi.org/10.1101/cshperspect.a000414
Hegde NV, Kariyawasam S, DebRoy C (2016) Comparison of antimicrobial resistant genes in chicken gut microbiome grown on organic and conventional diet. Vet Anim Sci 1–2:9–14. https://doi.org/10.1016/J.VAS.2016.07.001
Gueimonde M, Sánchez B, de los Reyes-Gavilán CG, Margolles A (2013) Antibiotic resistance in probiotic bacteria. Front Microbiol 4:202. https://doi.org/10.3389/fmicb.2013.00202
Hmani H, Daoud L, Jlidi M et al (2017) A Bacillus subtilis strain as probiotic in poultry: selection based on in vitro functional properties and enzymatic potentialities. J Ind Microbiol Biotechnol 44:1157–1166. https://doi.org/10.1007/s10295-017-1944-x
Lee J, Park I, Choi Y, Cho J (2012) Bacillus strains as feed additives: In vitro evaluation of its potential probiotic properties. Rev Colomb Cienc Pecu 25:577–585
Amiranashvili LL, Gagelidze NA, Varsimashvili KI et al (2016) Antimicrobial susceptibility and antibiotic resistance profiles of cultivable lactic acid bacteria from intestinal tract of domestic chickens collected in Adjara. Ann Agrar Sci 14:182–186. https://doi.org/10.1016/J.AASCI.2016.08.001
Prabhurajeshwar C, Chandrakanth RK (2017) Probiotic potential of Lactobacilli with antagonistic activity against pathogenic strains: An in vitro validation for the production of inhibitory substances. Biomed J 40:270–283. https://doi.org/10.1016/j.bj.2017.06.008
Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S et al (2012) Probiotic mechanisms of action. Ann Nutr Metab 61:160–174. https://doi.org/10.1159/000342079
Ben Lagha A, Haas B, Gottschalk M, Grenier D (2017) Antimicrobial potential of bacteriocins in poultry and swine production. Vet Res 48:22. https://doi.org/10.1186/s13567-017-0425-6
Józefiak D, Sip A (2013) Bacteriocins in poultry nutrition: a review. Ann Anim Sci 13:449–462. https://doi.org/10.2478/aoas-2013-0031
Acknowledgements
This work was supported by the Department of Biotechnology (DBT), Ministry of Science & Technology, Government of India (Sanction Order No. BT/IN/Indo-US/Foldscope/39/2015). We are thankful to the Dr. Peralam Yegneswaran Prakash, Associate Professor, Department of Microbiology, Kasturba Medical College, Manipal for his timely inputs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Research Involving Human and Animal Participants
This article does not involve human or animal experimentation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Priyodip, P., Balaji, S. Probiotic Validation of a Non-native, Thermostable, Phytase-Producing Bacterium: Streptococcus thermophilus. Curr Microbiol 77, 1540–1549 (2020). https://doi.org/10.1007/s00284-020-01957-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-01957-w