Abstract
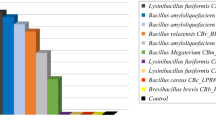
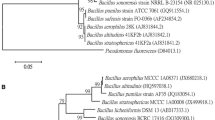
Four antagonists bacteria namely, Bacillus megaterium MB3, B. subtilis MB14, B. subtilis MB99 and B. amyloliquefaciens MB101 were able to produce chitinase, β-1,3-glucanase and protease in different range with the presence of Rhizoctonia solani cell wall as a carbon source. Amplification of chitinase (chiA) gene of 270 bp and β-1, 3-glucanase gene of 415 bp was given supportive evidence at molecular level of antibiosis. After in vitro screening, all antagonists were tested against R. solani under greenhouse conditions. Root treatment of Bacillus strains showed superior defense during pathogen suppression in terms of chitinase, glucanase, peroxidase, poly phenol oxidase, phenylalanine ammonia-lyase activity and total phenolic content in leaves of tomato. All these enzymes accumulated high in tomato leaves as compared to roots. Pathogenesis-related proteins and defense-related enzymes accumulation was directly correlated with plant protection and greenhouse results indicated that B. amyloliquefaciens MB101- and B. subtilis MB14-treated plants offered 69.76 and 61.51 % disease reductions, respectively, over the infected control. These results established that these organisms have the potential to act as biocontrol agents. This study could be highlighted a mutual importance of liquid formulation of antagonistic Bacillus spp. against root associated sclerotia former pathogen R. solani.



Similar content being viewed by others
References
Baysal Ö, Çalışkan M, Yeşilova Ö (2008) An inhibitory effect of a new Bacillus subtilis strain (EU07) against Fusarium oxysporum f. sp. radicis-lycopersici. Physiol Mol Plant Pathol 73:25–32
Boller T, Mauch F (1988) Colorimetric assay for chitinase. Methods Enzymol 161:430–435
Chandra A, Saxena R, Dubey A, Saxena P (2007) Change in phenylalanine ammonia lyase activity and isozyme patterns of polyphenol oxidase and peroxidase by salicylic acid leading to enhance resistance in cowpea against Rhizoctonia solani. Acta Physiol Plant 29:361–367
Choudhary DK, Johri BN (2009) Interactions of Bacillus spp. and plants with special reference to induced systemic resistance (ISR). Microbiol Res 164:493–513
Dickerson DP, Pascholati SF, Hagerman AE, Butler LG, Nicholson RL (1984) Phenylalanine ammonia-lyase and hydroxyl cinnamate: coA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol Plant Pathol 25:111–123
Goellner K, Conrath U (2008) Priming: it’s all the world to induced disease resistance. Eur J Plant Pathol 121:233–242
Hammerschmidt R, Nuckles EM, Kúc J (1982) Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol Plant Pathol 20:73–82
Huang CJ, Wang TK, Chung SC, Chen CY (2005) Identification of an antifungal chitinase from a potential biocontrol agent, Bacillus cereus 28-9. J Biochem Mol Biol 38:82–88
Inglis GD, Kawchuck LM (2002) Comparative degradation of oomycete, ascomycete, and basidiomycete cell walls by mycoparasitic and biocontrol fungi. Can J Microbiol 48:60–70
Jung WJ, Park RD, Mabood F, Souleimanov A, Donald LS (2011) Effects of Pseudomonas aureofaciens 63-28 on defense responses in soybean plants infected by Rhizoctonia solani. J Microbiol Biotechnol 21:379–386
Kumhar CK, Tripathi NN (2008) Biological management of root-rot of tomato caused by Rhizoctonia solani. Indian J Agr Res 42:1
Mayer AM, Harel E, Shaul RB (1965) Assay of catechol oxidase a critical comparison of methods. Phytochemistry 5:783–789
Nandakumar R, Babu S, Radjacommare R, Raguchander T, Samiyappan R (2002) Pseudomonas fluorescens mediated antifungal activity against Rhizoctonia solani causing sheath blight in rice. Phytopathol Mediterr 41:109–119
Pan SQ, Ye XS, Kúc J (1991) Association of β-1,3-glucanase activity and isoform pattern with systemic resistance to blue mould in tobacco induced by stem injection with Peronospora tabacina or leaf inoculation with tobacco mosaic virus. Physiol Mol Plant Pathol 39:25–39
Rajendran L, Samiyappan R (2008) Endophytic Bacillus species confer increased resistance in cotton against damping off disease caused by Rhizoctonia solani. J Plant Pathol 7:1–12
Ramaiah N, Hill RT, Chun J, Ravel J, Matte MH, Straube WL, Colwell RR (2000) Use of a chiA probe for detection of chitinase genes in bacteria from the Chesapeake Bay. FEMS Microb Ecol 34:63–71
Ramamoorthy V, Raguchander T, Samiyappan R (2002) Induction of defense-related proteins in tomato roots treated with Pseudomonas fluorescens Pf1 and Fusarium oxysporum f. sp. lycopersici. Plant Soil 239:55–68
Solanki MK, Kumar S, Pandey AK, Srivastava S, Singh RK, Kashyap PL, Srivastava AK, Arora DK (2012) Diversity and antagonistic potential of Bacillus spp. associated to the rhizosphere of tomato for the management of Rhizoctonia solani. Biocontrol Sci Technol 22:203–217
Solanki MK, Singh N, Singh RK, Singh P, Srivastava AK, Kumar S, Kashyap PL, Arora DK (2011) Plant defense activation and management of tomato root rot by a chitin-fortified Trichoderma/Hypocrea formulation. Phytoparasitica 39:471–481
Zieslin N, Ben–Zaken R (1993) Peroxidase activity and presence of phenolic substances in peduncles of rose flowers. Plant Physiol Biochem 31:333–339
Acknowledgments
This study was funded by the Indian Council of Agriculture Research (ICAR) by a network project “Application of Microorganisms in Agriculture and Allied Sectors” (AMAAS).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Solanki, M.K., Robert, A.S., Singh, R.K. et al. Characterization of Mycolytic Enzymes of Bacillus Strains and Their Bio-Protection Role Against Rhizoctonia solani in Tomato. Curr Microbiol 65, 330–336 (2012). https://doi.org/10.1007/s00284-012-0160-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-012-0160-1