Abstract
Purpose
Considerable amounts of injected immunoglobulin G-based therapeutic monoclonal antibodies, such as ramucirumab, are distributed into ascites. This study aimed to examine the effect of massive ascites on ramucirumab pharmacokinetics in patients with gastrointestinal cancers.
Methods
Population pharmacokinetic analysis of ramucirumab was performed using data on serum ramucirumab concentrations of 52 patients with gastrointestinal cancers, including 8 patients with massive ascites. The Bayesian method using the final population pharmacokinetic model was utilized to estimate trough ramucirumab concentrations after the first dose and at steady state.
Results
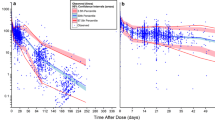
Population pharmacokinetic analysis revealed that massive ascites as well as body weight were influencing factors for ramucirumab clearance. The estimated ramucirumab clearance was significantly higher in patients with massive ascites than in those with no/mild ascites (0.020 ± 0.004 versus 0.013 ± 0.004 L/h, P < 0.001). The estimated trough ramucirumab concentrations were significantly lower in patients with massive ascites than in those with no/mild ascites after the first dose (26.4 ± 6.8 versus 36.1 ± 7.1 μg/mL, P < 0.001) and at steady state (41.4 ± 16.3 versus 65.9 ± 18.0 μg/mL, P < 0.001).
Conclusion
In the present study, the presence of massive ascites affected the pharmacokinetics of ramucirumab in patients with gastrointestinal cancers. Our results suggest that dose optimization of ramucirumab may be necessary in patients with massive ascites due to higher ramucirumab clearance.



Similar content being viewed by others
Data availability
The study data are available from the corresponding author on reasonable request.
References
Fuchs CS, Tomasek J, Yong CJ et al (2014) Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383:31–39. https://doi.org/10.1016/S0140-6736(13)61719-5
Wilke H, Muro K, Van Cutsem E et al (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15:1224–1235. https://doi.org/10.1016/S1470-2045(14)70420-6
Iwasa S, Bando H, Piao Y et al (2022) The clinical position of ramucirumab-containing regimens for advanced gastric cancer: a review of clinical trial data. Futur Oncol 18:2709–2721. https://doi.org/10.2217/fon-2022-0281
European medicines agency (EMA) (2022) Cyramza-epar product information. http://www.ema.europa.eu. Accessed 4 Mar, 2023
Tabernero J, Ohtsu A, Muro K et al (2017) Exposure-response analyses of ramucirumab from two randomized, phase III trials of second-line treatment for advanced gastric or gastroesophageal junction cancer. Mol Cancer Ther 16:2215–2223. https://doi.org/10.1158/1535-7163.MCT-16-0895
O’Brien L, Westwood P, Gao L, Heathman M (2017) Population pharmacokinetic meta-analysis of ramucirumab in cancer patients. Br J Clin Pharmacol 83:2741–2751. https://doi.org/10.1111/bcp.13403
Cavazzoni E, Bugiantella W, Graziosi L et al (2013) Malignant ascites: pathophysiology and treatment. Int J Clin Oncol 18:1–9. https://doi.org/10.1007/s10147-012-0396-6
Becker G, Galandi D, Blum HE (2006) Malignant ascites: systematic review and guideline for treatment. Eur J Cancer 42:589–597. https://doi.org/10.1016/j.ejca.2005.11.018
Kaneko T, Doki K, Yamada T et al (2021) Bevacizumab distribution into ascitic fluid decreases serum drug exposure. Ther Drug Monit 43:813–814. https://doi.org/10.1097/FTD.0000000000000926
Kaneko T, Doki K, Yamada T et al (2022) Distribution of therapeutic monoclonal antibodies into ascites in advanced gastric cancer patients with peritoneal metastasis: case reports and literature review. Cancer Chemother Pharmacol 90:421–426. https://doi.org/10.1007/s00280-022-04479-3
Matsumoto H, Kawazoe A, Shimada K et al (2018) A retrospective study of the safety and efficacy of paclitaxel plus ramucirumab in patients with advanced or recurrent gastric cancer with ascites. BMC Cancer 18:120. https://doi.org/10.1186/s12885-018-4057-7
Nakajima TE, Yamaguchi K, Boku N et al (2020) Randomized phase II/III study of 5-fluorouracil/l-leucovorin versus 5-fluorouracil/l-leucovorin plus paclitaxel administered to patients with severe peritoneal metastases of gastric cancer (JCOG1108/WJOG7312G). Gastric Cancer 23:677–688. https://doi.org/10.1007/s10120-020-01043-x
Tabernero J, Ohtsu A, Muro K et al (2015) Exposure-response (E-R) relationship of ramucirumab (RAM) from two global, randomized, double-blind, phase 3 studies of patients (Pts) with advanced second-line gastric cancer. J Clin Oncol 33:abstr121. https://doi.org/10.1200/jco.2015.33.3_suppl.121
Kim TY, Yen CJ, Al-Batran SE et al (2018) Exposure–response relationship of ramucirumab in East Asian patients from RAINBOW: a randomized clinical trial in second-line treatment of gastric cancer. Gastric Cancer 21:276–284. https://doi.org/10.1007/s10120-017-0737-2
Cohn AL, Yoshino T, Heinemann V et al (2017) Exposure–response relationship of ramucirumab in patients with advanced second-line colorectal cancer: exploratory analysis of the RAISE trial. Cancer Chemother Pharmacol 80:599–608. https://doi.org/10.1007/s00280-017-3380-z
de Wit R, Powles T, Castellano D et al (2022) Exposure-response relationship of ramucirumab in RANGE, a randomized phase III trial in advanced urothelial carcinoma refractory to platinum therapy. Br J Clin Pharmacol 88:3182–3192. https://doi.org/10.1111/bcp.15233
Smit EF, Garon EB, Reck M et al (2018) Exposure–response relationship for ramucirumab from the randomized, double-blind, phase 3 REVEL trial (docetaxel versus docetaxel plus ramucirumab) in second-line treatment of metastatic non-small cell lung cancer. Cancer Chemother Pharmacol 82:77–86. https://doi.org/10.1007/s00280-018-3560-5
Funding
This research was supported in part by a Grant-in-Aid for Scientific Research (C) (Grant No. 22K06718) awarded to K. Doki by the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical approval
This study was approved by the Ethics Committee of University of Tsukuba Hospital (ethical approval No. R01-343).
Consent to participate
Written informed consent to participate was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaneko, T., Doki, K., Yamada, T. et al. Effect of massive ascites on ramucirumab pharmacokinetics in patients with gastrointestinal cancers: a population pharmacokinetic analysis. Cancer Chemother Pharmacol 92, 271–278 (2023). https://doi.org/10.1007/s00280-023-04568-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-023-04568-x