Abstract
Background
Oral fluoropyrimidine plus cisplatin is often not tolerated by patients with severe peritoneal metastases of gastric cancer. Combination of 5-fluorouracil (5-FU), l-leucovorin (l-LV), and paclitaxel (FLTAX) has promising activity for such patients. We conducted a phase II/III study comparing FLTAX with 5-FU/l-LV.
Methods
Eligibility criteria included: unresectable or recurrent gastric adenocarcinoma; 20–75 years; performance status (PS) 0–2; peritoneal metastases + ; massive ascites and/or inadequate oral intake; no prior chemotherapy. Patients were randomly assigned to receive 5-FU/l-LV or FLTAX. The primary endpoint of phase III was overall survival: UMIN000010949.
Results
We enrolled 101 patients. Early deaths occurred in patients with PS 2 having massive ascites and inadequate oral intake simultaneously; the protocol was amended to exclude such patients. Median survival times were 6.1 and 7.3 months for the 5-FU/l-LV and the FLTAX arms, respectively (HR 0.792; 80% CI 0.596–1.053; one-sided p = 0.1445). FLTAX arm had longer progression-free survival (PFS) [1.9 vs 5.4 months (HR 0.64; 95% CI, 0.43–0.96; p = 0.029)]. Grade 3/4 adverse events such as leucopenia and anorexia were more frequently observed in the 5-FU/l-LV arm. In the 5-FU/l-LV arm, two deaths were treatment-related. In the 5-FU/l-LV and FLTAX arms, 12 and 3 deaths occurred within 30 days after the last protocol treatment, respectively.
Conclusions
Chemotherapy was indicated for patients with severe peritoneal metastases excluding patients with PS 2 having massive ascites and inadequate oral intake simultaneously. FLTAX did not confer a significant survival benefit but may be preferred because of longer PFS and acceptable toxicity.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is the third leading cause of death from cancer worldwide with the highest age-standardized incidence in Eastern Asia [1]. The prognosis of unresectable or recurrent advanced disease is unacceptable [median survival time (MST), approximately 10–14 months] [2,3,4,5]. The peritoneum is the most frequent site of GC metastases [6] (20–40%) [4, 7]. However, patients with severe peritoneal metastases have poor prognoses and quality of life (QOL), despite chemotherapy [8,9,10], and have, therefore, been excluded from clinical trials designed to establish new standard chemotherapy regimens.
Continuous infusion of 5-fluorouracil [5-FU] (5-FU ci) is often administered in Japan to patients with GC with peritoneal metastases (GC-PM) according to the JCOG0106 trial conducted by the Japan Clinical Oncology Group (JCOG), demonstrating that methotrexate and 5-FU sequential therapy are not superior to 5-FU ci in chemotherapy-naïve patients with peritoneal metastases, excluding those with massive ascites [11]. Furthermore, the ISO-5FU study shows that a weekly bolus of 5-FU/l-leucovorin [l-LV] was noninferior to S-1 in patients with advanced GC and that S-1 was noninferior to 5-FU ci in the JCOG9912 trial [12, 13]. Thus, in Japan, bolus 5-FU/l-LV is most often administered to patients with severe GC-PM associated with massive ascites or inadequate oral intake.
Paclitaxel can be administered to such patients, and it showed promising efficacy compared with the best available 5-FU regimen as the second-line therapy for patients with GC-PM [median progression-free survival (PFS) and MST were 3.7 months and 7.7 months, respectively] [14]. Since paclitaxel has a bulky molecular structure, high molecular weight, and an affinity for binding proteins found at high levels in the peritoneal cavity, especially in malignant effusions, it has been reported that its intraperitoneal clearance is extraordinarily low [15]. Moreover, paclitaxel has been reported its synergistic effect in vitro when followed by 5-FU [16], and these 2 drugs are relatively free of overlapping toxic effects. We developed 5-FU/l-LV plus paclitaxel (FLTAX) therapy for such patients, which involves weekly administration without requiring hydration or oral agents [17, 18]. Toxicity of FLTAX is mild, and doses were lower than the recommended dose for patients with advanced GC without severe PM.
We, therefore, conducted a randomized phase II/III trial of 5-FU/l-LV vs FLTAX of patients with severe GC-PM as an intergroup study of the JCOG and West Japan Oncology Group (WJOG) (JCOG1108/WJOG7312G).
Methods
Patients
We conducted a randomized phase II/III trial at 43 institutions in Japan. Inclusion criteria: histologically proven gastric adenocarcinoma; unresectable or recurrent disease; age 20–75 years; Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–2; severe PM confirmed using barium enema, computed tomography (CT) or clinical diagnosis, massive ascites throughout the abdominal cavity, and/or inadequate oral intake requiring an intravenous drip infusion or adequate hydration [amended protocol (September 2015) excluded patients with ECOG PS 2 having massive ascites and inadequate oral intake simultaneously, because of the high proportion of early deaths]; previously untreated disease; recurrent disease diagnosed ≥ 6 months after completion of adjuvant chemotherapy; HER2 untested or negative in case of tested; adequate organ function (neutrophils ≥ 1500/mm3, hemoglobin ≥ 8.0 g/dL, platelets ≥ 100,000/mm3, aspartate aminotransferase and alanine aminotransferase ≤ 100 IU/L, total bilirubin ≤ 1.5 mg/dL, and serum creatinine ≤ 2.0 mg/dL); and written informed consent. Exclusion criteria: active concomitant malignancy, active infection, uncontrolled diabetes, uncontrolled heart disease, pulmonary fibrosis, massive pleural effusion, symptomatic brain metastases, and pregnancy or lactation. Ascites: massive, extending throughout the abdominal cavity; moderate, either mild or massive; mild, located only in the upper or lower abdominal cavity; no ascites, ascites not detected by CT scan.
The review committees of the JCOG and WJOG and the institutional review boards of all participating institutions approved the study protocol, which was conducted according to the Declaration of Helsinki and Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects. The JCOG Data and Safety Monitoring Committee (DSMC) and the WJOG DSMC monitored safety and progress. This study was registered with the UMIN-CTR (UMIN000010949).
Randomization
Each eligible patient was randomly assigned to receive 5-FU/l-LV or FLTAX using the minimization method with a random component [19] with an algorithm (concealed to investigators) that balanced institution, ECOG PS (0, 1, or 2), and both of massive ascites and inadequate oral intake or either. Randomization was performed by web-based system at the JCOG Data Center from the institutions in JCOG or the WJOG Data Center from the institutions in WJOG. This was an open-label trial.
Treatment
Protocols
5-FU/l-LV: 600 mg/m2 5-FU and 250 mg/m2l-LV infusions on days 1, 8, 15, 22, 29, and 36, repeated every 8 weeks. 5-FU was intravenously administered as a bolus 60 min after a 120-min intravenous infusion of l-LV. FLTAX: 500 mg/m2 5-FU, 250 mg/m2l-LV, and 60 mg/m2 paclitaxel administered on days 1, 8, and 15, repeated every 4 weeks. Paclitaxel was administered as a 60-min intravenous infusion followed by 5-FU as an intravenous bolus. A 120-min intravenous infusion of l-LV was started simultaneously. Prophylactic treatment for potential paclitaxel hypersensitivity (8 mg dexamethasone, 50 mg ranitidine, and 10 mg of chlorpheniramine) was administered before paclitaxel.
Modifications of treatments
Subsequent treatment cycles were delayed until nonhematological toxicities such as diarrhea, stomatitis, pneumonitis, and infection had recovered to grade 1 or less, according to the Common Terminology Criteria for Adverse Events (CTCAE) (version 4.0); no infection-associated fever; neutrophils ≥ 1500/mm3; platelets ≥ 75,000/mm3; aspartate aminotransferase and alanine aminotransferase each ≤ 100 IU/L; total bilirubin ≤ 2.0 mg/dL; and creatinine ≤ 2.0 mg/dL. For FLTAX, paclitaxel was skipped if patients experienced grade 3 or more neuropathy and allergic reactions. Doses of each treatment were reduced if the events occurred during the previous cycle: grade-4 neutropenia; grade-3 or -4 thrombocytopenia; total bilirubin > 3.0 mg/dL; creatinine > 2.0 mg/dL; grade-3 febrile neutropenia; grade-3 infection; grade-2 or -3 diarrhea or stomatitis; or grade-2 pneumonitis. Doses of FLTAX were reduced if grade-3 fatigue or grade-3 neuropathy occurred. Paclitaxel was discontinued if grade-2 or -3 allergic reactions occurred twice despite hypersensitivity prophylaxis. Treatment was terminated when (1) disease progression was clinically diagnosed or by imaging; (2) life-threatening adverse events; (3) treatment cycle delayed because of any adverse event persisting for 28 days; (4) adverse event requiring subsequent dose reduction after a second reduction; or (5) patient refused further treatment.
Evaluations
Physical examinations and laboratory tests were performed weekly. Adverse events were classified according to CTCAE (version 4.0). Serious adverse events: death within 30 days from the last treatment; death > 30 days after discontinuing treatment that was possibly, probably, or definitely attributable to treatment; grade-4 nonhematological adverse events; and unknown grades 1–3 adverse events requiring hospitalization. The JCOG and WJOG DSMC reviewed all serious adverse events and judged whether they were attributable to treatment. Diagnosis of disease progression guided decisions to discontinue protocol treatment according to the patient’s general condition, symptoms, physical examination, or CT (performed every 2 months).
QOL surveys using the EuroQol EQ-5D-3L questionnaire were repeated for all patients capable of responding before treatment and every 3 months until 12 months after initiating treatment [20].
Outcomes
Endpoints
This study had specified primary and secondary endpoints for each phase. Coprimary phase II endpoints were the proportion of patients continuing treatment at 8 weeks in the FLTAX arm and the MST of each arm. Secondary phase II endpoints were the proportion of patients continuing treatment at 8 weeks in the 5-FU/l-LV arm and adverse events in each arm. The primary phase III endpoint was overall survival (OS). Secondary phase III endpoints were PFS, time-to-treatment failure, adverse event incidence, proportion of improvement in oral intake of patients with inadequate oral intake on enrollment, ascites drainage-free survival of patients with ascites on enrollment, proportion of ascites response and ascites control on enrollment, proportion of patients surviving without deteriorated QOL (assessed pretreatment and every 3 months) 12 months from initiating treatment, and dose intensity.
Definition of endpoints
OS was defined as days from the date of randomization to the date of death from any cause or censored at the last contact date confirming survival. PFS was defined as days from the date of randomization to the date of disease progression or death from any cause. Without documented disease progression and the patient was alive, data on PFS were censored on the date that the absence of progression was confirmed. Time-to-treatment failure was defined as days from the date of randomization to the date of treatment failure, including disease progression, adverse events, or death from any cause. If the patient had not terminated treatment, data on time-to-treatment failure were censored on the date of the last contact date confirming continuing treatment. Ascites drainage-free survival was defined as days from the date of randomization to the date of drainage, cell-free and concentrated ascites reinfusion, or death from any cause.
Best ascites response was evaluated in patients who initially had ascites as follows: complete response (CR), disappearance of ascites; partial response (PR), decrease in severity of ascites by at least one level as described above; stable disease (SD), other than CR, PR, or progressive disease (PD); PD, increase by at least one level; and not evaluable (NE), ascites drained during protocol treatment; or no CT. Proportion of ascites response: number of patients with best ascites response of CR or PR divided by the number of patients who initially had ascites. Proportion of ascites control: number of patients with best ascites response of CR, PR, or SD divided by the number of patients who initially had ascites. A patient with inadequate oral intake at baseline was judged to be able to resume oral intake if intravenous infusion for 7 days was not required.
Statistical analysis
There was no basis for firm expectations in either cohort with severe GC-PM. Therefore, the plan was to make decisions to proceed from phase II to phase III according to (i) MST of each arm, threshold value at 3 months, expected value at 6 months, one-sided alpha 0.05, and power 90%; and (ii) proportion of treatment success at week 8 in the FLTAX arm, threshold 30% and expected value, 50%, one-sided alpha 0.05, and power 80%. Thus, the planned sample size was 100 patients for phase II. In phase III, we planned to investigate the efficacy of FLTAX vs 5-FU/l-LV associated with OS to establish standard chemotherapy for patients with severe GC-PM.
A 6-month MST was assumed for the 5-FU/l-LV arm. To observe 298 deaths, the initial total sample size was 330, which allowed detection of a 2-month longer MST in the FLTAX arm (hazard ratio [HR], 0.75); one-sided alpha 0.05; power ≥ 80%; accrual, 3.5 years; and follow-up 1 year. An interim analysis of phase III was planned when enrollment in phase II was completed. Multiplicity was adjusted using the O’Brien and Fleming alpha spending function of Lan and DeMets [21].
In contrast, the eligibility criteria were amended and termination rules for safety were modified after 59 patients were enrolled. Early deaths occurred in patients with ECOG PS 2 having massive ascites and inadequate oral intake simultaneously, and thus, the DSMC recommended protocol amendment to exclude such patients. In August 2017, 102 patients were enrolled in phase II, and the protocol was amended before the time scheduled for interim analysis because of poor accrual. Interim analysis was not performed, and the amended sample size (n = 102) for the final analysis in phase III allowed detection of 2-month longer MST in the FLTAX arm, one-sided alpha 0.1, and power 57%. Totally, we performed the pre-planned analysis for phase II, and the final analysis for phase III based on the amended sample size and statistical setting.
Time-to-event type endpoints were determined using the Kaplan–Meier method. OS was analyzed using the log-rank test stratified by ECOG PS (0–1/2) and both of massive ascites and inadequate oral intake or either. The HR and confidence interval (CI) was estimated using the stratified Cox’s proportional-hazard model. Other time-to-event endpoints were analyzed using the log-rank test, and HR and CI were estimated using the Cox’s proportional-hazard model. The proportion of patients surviving without QOL deterioration at each assessment were analyzed using a logistic regression model with baseline scores as covariates; and odds ratios (ORs) and CIs were estimated. Repeated-measures analysis was performed to estimate adjusted scores of EQ-5D-3L including treatment, time, and time–treatment interactions as fixed effects. Other binary data were analyzed using Fisher’s exact test.
Subset analysis of OS was planned and performed using a univariable Cox’s proportional-hazard model with the variables sex, ECOG PS (0 or 1 vs 2), age (≤ 64 vs ≥ 65 years), metastatic sites except the peritoneum (0 or 1 vs ≥ 2), histological type (differentiated vs undifferentiated adenocarcinoma), disease (unresectable vs recurrent), Glasgow Prognostic Score (GPS) (0 vs 1 vs 2) [22], and factors associated with severe PM (massive ascites only vs inadequate oral intake only vs both; either vs both). Post hoc multivariable analysis (Cox’s proportional-hazard model) was conducted to identify prognostic factors.
Efficacy analyses were performed with intention-to-treat, and safety analysis was performed for all patients who initiated treatment. Statistical analysis was performed using SAS version 9.4 (Cary, NC, USA). Unless otherwise specified, two-sided P values and 95% CIs are presented.
Results
Patients
Between June 2013 and February 2017, 102 patients were enrolled, one revoked consent. Fifty-one and fifty patients were allocated to the 5-FU/l-LV and FLTAX arms, respectively (Fig. 1). Two patients included in the FLTAX arm did not undergo treatment. Baseline characteristics were well balanced between the arms (Table 1). Approximately 20% of the patients had ECOG PS 2. Undifferentiated tumors were more common than differentiated tumors in both arms, 18.8% of patients had massive ascites and inadequate oral intake, massive ascites was more common than inadequate oral intake, and approximately 50% of patients in both arms had adequate baseline oral intake.
Feasibility and safety
The data that follow are included in Table 2. The percentages of patients continuing treatment at 8 weeks were 45.1% (95% CI 31.3–59.7) and 66.7% (95% CI 51.6–79.6) in the 5-FU/l-LV and FLTAX arms, respectively. Relative mean dose intensities were as follows: 5-FU in the 5-FU/l-LV arm, 71.4% [standard deviation (SD), 24.3%]; 5-FU in the FLTAX arm, 83.0% (SD, 17.3%); and paclitaxel in the FLTAX arm, 82.5% (SD, 17.4%).
Safety analysis revealed that grade-3 or -4 adverse events were experienced by 78.4% and 77.1% of patients in the 5-FU/l-LV and FLTAX arms, respectively. Grade-3 or -4 adverse events were experienced by 9 patients (90.0%) with massive ascites and inadequate oral intake in the 5-FU/l-LV arm; 6 patients (75.0%) in the FLTAX arm; 12 patients (92.3%), PS 2, in the 5-FU/l-LV arm; and 12 patients (92.3%) in the FLTAX arm. Grade-3 or -4 adverse events that occurred in > 15% of patients were more frequent in the 5-FU/l-LV arm, except anemia.
Nine patients in each arm discontinued treatment because of adverse events or refusal related to toxicity. Two patients in the 5-FU/l-LV arm died because of treatment-related causes. Four deaths (7.8%) occurred within 30 days of randomization in the 5-FU/l-LV arm and one (2.1%) in the FLTAX arm. Twelve patients (23.5%) died within 30 days of the last protocol treatment in the 5-FU/l-LV arm and three (6.3%) in the FLTAX arm.
Efficacy
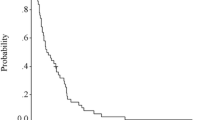
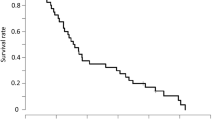
Upon primary analysis (February 2018), 91 events occurred. The median follow-up was 6.4 months (range 0.2–48.1 months). MSTs were 6.1 (95% CI 3.4–7.8) and 7.3 (95% CI 5.0–10.6) months in the 5-FU/l-LV and FLTAX arms, respectively, (HR 0.79; 80% CI 0.60–1.05; one-sided p = 0.14) (Fig. 2a), indicating that the decision to proceed to phase III was supported by the significant proportion of treatment success at week 8 in the FLTAX arm in the phase II analysis, although the primary phase III endpoint was not met. The MSTs of the patients with PS 2, massive ascites, and inadequate oral intake, who were excluded after the protocol amendment, were 0.8 months and 2.2 months in the 5-FU/l-LV and FLTAX arms, respectively.
Median PFS times were 1.9 months (95% CI 1.5–3.5) and 5.4 (95% CI 2.6–6.9) months in the 5-FU/l-LV and FLTAX arms, respectively (HR 0.64; 95% CI 0.43–0.96; p = 0.029) (Fig. 2b). The median time-to-treatment failure was 1.9 months (95% CI 1.3–3.1) and 5.1 (95% CI 2.0–6.9) months in the 5-FU/l-LV and FLTAX arms, respectively (HR 0.64; 95% CI 0.43–0.96; p = 0.030).
Oral intake by patients with initial inadequate oral intake showed improvements of 32.0% (95% CI 15.0–53.5) and 37.0% (95% CI 19.4–57.6) (p = 0.78) in the 5-FU/l-LV and FLTAX arms, respectively. Median ascites drainage-free survival times of patients with initial ascites were 4.7 months (95% CI 2.0–7.0) and 6.5 (95% CI 3.5–8.3) months in the 5-FU/l-LV and FLTAX arms, respectively (HR 0.91; 95% CI 0.60–1.39; p = 0.66). Ascites responses occurred in 27.5% (95% CI 15.9–41.7) and 36.2% (95% CI 22.7–51.5) of patients in the 5-FU/l-LV and FLTAX arms, respectively (p = 0.39). Ascites was controlled by 58.8% (95% CI 44.2–72.4) and 63.8% (95% CI 48.5–77.3) in the 5-FU/l-LV and FLTAX arms, respectively (p = 0.68).
A higher proportion of patients survived without deterioration of QOL in the FLTAX arm at all times (Fig. 3a). EQ-5D-3L scores were 0.69 ± 0.17 and 0.68 ± 0.17 in the 5-FU/l-LV and FLTAX arms at baseline, respectively, and 0.71 ± 0.28 and 0.72 ± 0.23 in the 5-FU/l-LV and FLTAX arms 3 months after initiating treatment, respectively (Fig. 3b).
Second-line chemotherapy
Second-line chemotherapy was administered to 29 (56.9%) and 28 (56.0%) patients in the 5-FU/l-LV and FLTAX arms, respectively (Supplementary Table 1). Regimens containing taxane were administered to 25 and 4 patients in the 5-FU/l-LV and FLTAX arms, respectively. Regimens containing oxaliplatin were administered to 3 and 10 patients in the 5-FU/l-LV and FLTAX arms, respectively.
Subset analysis
Subset analyses of OS are described in Table 3. Prognosis was significantly worse for patients with PS 2, unresectable disease, or GPS 2.
Subset analyses for comparisons of efficacy, in terms of OS, between the protocol treatments were also performed (Fig. 4). Prognosis of female patients, with PS 2, two or more metastatic sites, histologically undifferentiated adenocarcinoma, unresectable disease, or with GPS 2 exhibited better trends in the FLTAX arm than in the 5-FU/l-LV arm.
Discussion
Here, we report the first randomized phase II/III study of severe GC-PM. Although FLTAX did not show significant superiority to 5-FU/l-LV in terms of OS, PFS and QOL in the FLTAX arm were favorable compared to those in the 5-FU/l-LV arm. The phase II MSTs were at least 3 months, suggesting the suitability of FLTAX for the first-line chemotherapy. However, there was a high percentage of early deaths of patients with PS 2, massive ascites, and inadequate oral intake, and consequently their MSTs were shorter than 3 months. We, therefore, decided during the study to exclude such patients, because these findings indicated that chemotherapy was contraindicated and that best supportive care should serve as standard management.
Retrospective studies conducted in Japan show that 28–66% of patients with severe GC-PM receive the second-line chemotherapy [8,9,10] compared with > 75% of patients with advanced GC [3, 4, 7, 13]. Thus, effective and safe first-line chemotherapy is yet to be identified. Accordingly, we developed FLTAX. In this study, we administered the second-line chemotherapy to 56.9% and 56.0% of patients treated with 5-FU/l-LV and FLTAX, respectively.
Advanced GC patients with better baseline QOL achieve longer OS and PFS [11, 13, 23]. For example, the baseline QOL score is 0.73 ± 0.19 (mean, SD) [23]. Here, we show that the QOL scores of the treatment groups were not significantly different (Fig. 3b) and are lower compared with those of other clinical trials of treatments for advanced GC in general, suggesting that poor prognosis of our patients was associated with poor baseline QOL. We speculate that prompt prevention to mitigate the deterioration of QOL may confer a benefit (Fig. 3a, b).
Grade-3 or -4 neutropenia was more frequently observed in the 5-FU/l-LV arm, and the two treatment-related deaths were caused by febrile neutropenia and sepsis. The relative dose intensity of 5-FU was higher in the FLTAX arm, suggesting that severe neutropenia associated with the 5-FU/l-LV arm may be attributed to skipped, delayed, or discontinued treatment early during first-line treatment. These possibilities may have prevented prompt efforts to ameliorate disease progression and improve QOL. Thus, deaths within 30 days of randomization and within 30 days of the last treatment occurred more frequently in the 5-FU/l-LV arm. In contrast, patients treated with FLTAX with factors indicating poor prognosis such as PS 2, unresectable disease, and GPS 2 had a trend of longer OS (Fig. 4). Moreover, for patients in poor condition, such as those with massive ascites and inadequate oral intake, severe toxicities occurred more often in those treated with 5-FU/l-LV. Thus, FLTAX may be more beneficial for patients with poor prognostic factors.
This study had certain limitations. Insufficient patient accrual may be attributed to inaccurate estimation of the number of eligible patients. Although we presumed that 16% of patients with metastatic GC were eligible according to retrospective data [9], the actual value was 3%. Moreover, the number of patients with severe GC-PM was small. Therefore, we accepted a one-sided alpha error 0.1, which is significantly higher compared with those of other randomized phase II/III trials, to establish a new standard regimen for such patients in the small-sized phase III part. Another limitation was that the study was conducted in Japan, limiting the generalization of our findings.
5-FU/l-LV/oxaliplatin (FOLFOX), which does not require hydration and does not include oral agents, was not approved for GC in Japan until February 2017. We, therefore, developed FLTAX as the most promising regimen for patients with severe GC-PM. We are evaluating the feasibility and efficacy of FOLFOX in a phase II study (WJOG10517G: jRCTs041180007) of patients with GC using the criteria of the present study.
Chemotherapy was indicated for patients with severe GC-PM excluding patients with PS 2 having massive ascites and inadequate oral intake simultaneously. Although OS associated with FLTAX was not significantly superior to that of 5-FU/l-LV, FLTAX may serve as an alternative, because it achieved longer PFS with comparable toxicity and QOL. Further development of effective regimens is required for patients with severe GC-PM with poor prognoses and poor QOL.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, ToGA Trial Investigators, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-esophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial. Lancet. 2010;376:687–97.
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21.
Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141–8.
Fuchs CS, Shitara K, Di Bartolomeo M, Lonardi S, Al-Batran SE, Van Cutsem E, RAINFALL Study Group, et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomized, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:420–35.
Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Japan Clinical Oncology Group, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–62.
Koizumi W, Kim YH, Fujii M, Kim HK, Imamura H, Lee KH, JACCRO, and KCSG Study Group, et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol. 2014;140:319–28.
Iwasa S, Nakajima TE, Nakamura K, Takashima A, Kato K, Hamaguchi T, et al. Systemic chemotherapy for peritoneal disseminated gastric cancer with inadequate oral intake: a retrospective study. Int J Clin Oncol. 2011;16:57–62.
Iwasa S, Nakajima TE, Nakamura K, Takashima A, Kato K, Hamaguchi T, et al. First-line fluorouracil-based chemotherapy for patients with severe peritoneal disseminated gastric cancer. Gastric Cancer. 2012;15:21–6.
Hara H, Kadowaki S, Asayama M, Ooki A, Yamada T, Yoshii T, et al. First-line bolus 5-fluorouracil plus leucovorin for peritoneally disseminated gastric cancer with massive ascites or inadequate oral intake. Int J Clin Oncol. 2018;23:275–80.
Shirao K, Boku N, Yamada Y, Yamaguchi K, Doi T, Goto M, et al. Randomized Phase III study of 5-Fluorouracil continuous infusion vs. sequential methotrexate and 5-fluorouracil therapy in far advanced gastric cancer with peritoneal metastasis (JCOG0106). Jpn J Clin Oncol. 2013;43:972–80.
Sawaki A, Yamaguchi K, Nabeya Y, Sakai Y, Osanai H, Denda T, et al. 5-FU/l-LV (RPMI) versus S-1 as first-line therapy in patients with advanced gastric cancer: a randomized phase III non-inferiority trial (ISO-5FU10 Study Group trial). Eur J Cancer Suppl. 2009;7:364.
Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomized phase 3 study. Lancet Oncol. 2009;10:1063–9.
Nishina T, Boku N, Gotoh M, Shimada Y, Hamamoto Y, Yasui H, Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group, et al. Randomized phase II study of second-line chemotherapy with the best available 5-fluorouracil regimen versus weekly administration of paclitaxel in far advanced gastric cancer with severe peritoneal metastases refractory to 5-fluorouracil-containing regimens (JCOG0407). Gastric Cancer. 2016;19:902–10.
Kobayashi M, Sakamoto J, Namikawa T, Okamoto K, Okabayashi T, Ichikawa K, et al. Pharmacokinetic study of paclitaxel in malignant ascites from advanced gastric cancer patients. World K Gastroenterol. 2006;12:1412–5.
Kano Y, Akutsu M, Tsunoda S, Ando J, Matsui J, Suzuki K, et al. Schedule-dependent interaction between paclitaxel and 5-fluorouracil in human carcinoma cell lines in vitro. Br J Cancer. 1996;74:704–10.
Matsubara J, Shimada Y, Kato K, Nagai Y, Iwasa S, Nakajima TE, et al. Phase II study of bolus 5-fluorouracil and leucovorin combined with weekly paclitaxel as first-line therapy for advanced gastric cancer. Oncology. 2011;81:291–7.
Iwasa S, Goto M, Yasui H, Nishina T, Takahari D, Nakayama N, et al. Multicenter feasibility study of combination therapy with fluorouracil, leucovorin and paclitaxel (FLTAX) for peritoneal disseminated gastric cancer with massive ascites or inadequate oral intake. Jpn J Clin Oncol. 2012;42:787–93.
Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–15.
The Economics Network. EQ-5D index calculator. [Cited 2019 May 2]. https://www.economicsnetwork.ac.uk/health/EQ_5D_index_calculator.xls
Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–63.
Yuan SQ, Nie RC, Chen YM, Qiu HB, Li XP, Chen XJ, et al. Glasgow Prognostic Score is superior to ECOG PS as a prognostic factor in patients with gastric cancer with peritoneal seeding. Oncol Lett. 2018;15 4193–4200.
Abdel-Rahman O. Prognostic impact of baseline quality of life status among patients with advanced gastric cancer; results from two randomized studies. Expert Rev Pharmacoecon Outcomes Res. 2019;22:1–5.
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, V325 Study Group, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 study group. J Clin Oncol. 2006;24:4991–7.
Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–9.
Acknowledgments
We thank the patients and their families, our collaborators who contributed to the study and recruited patients, the members of the JCOG/WJOG Data Center and JCOG/WJOG Operations Office for their support, and ASCA Corporation for editing a draft of this manuscript.
Funding
The study was supported in part by the National Cancer Research and Development Fund (26-A-4, 29-A-3), a Grant-in-Aid for Clinical Cancer Research (H26-144) from the Ministry of Health, Labour and Welfare of Japan, and by the Japan Agency for Medical Research and Development (AMED) (Grant Numbers JP16ck0106139 and JP18ck0106351). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design, and conduct. Material preparation, data collection, and analysis were performed by Junki Mizusawa, Hiroshi Katayama, and Shinichiro Nakamura. The first draft of the manuscript was written by Takako Eguchi Nakajima, and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Nakajima reports grants from AMED, during the conduct of the study; grants and personal fees from Taiho Pharmaceutical Co., Ltd., grants and personal fees from Merck Serono Co., Ltd., grants and personal fees from Chugai Pharmaceutical Co., Ltd., grants and personal fees from Takeda Pharmaceutical Co., Ltd., grants and personal fees from Sanofi K.K., grants from Daiichi Sankyo Co., Ltd., grants and personal fees from Eli Lilly Japan K.K., grants and personal fees from Nippon Kayaku Co., Ltd., grants and personal fees from Ono Pharmaceutical Co., Ltd., grants from Astellas Pharma Inc., grants and personal fees from Sumitomo Dainippon Pharma Co., Ltd., grants from Eisai Co., grants and personal fees from MSD K.K., grants from Solasia Pharma K.K., grants from AstraZeneca K.K., personal fees from Sawai Pharmaceutical Co., personal fees from Bayer Yakuhin, Ltd., personal fees from Bristol-Myers Squibb, personal fees from Mochida Pharmaceutical Co., Ltd., personal fees from Kyowa Kirin Co., Ltd., personal fees from Maruho Co., Ltd., personal fees from Teijin Pharma Limited, and grants from AMED unrelated to the present study. Dr. Yamaguchi reports grants from AMED, during the conduct of the study; grants and personal fees from Taiho Pharmaceutical Co., Ltd., grants and personal fees from Chugai Pharmaceutical Co., Ltd., personal fees from Merck Serono Co., Ltd., personal fees from Takeda Pharmaceutical Co., Ltd., grants and personal fees from Daiichi Sankyo Co., Ltd., grants and personal fees from Ono Pharmaceutical Co., Ltd., personal fees from Elli Lilly Japan K.K., personal fees from Bristol-Myers Squibb, grants from Sumitomo Dainippon Pharma Co., Ltd., grants from Boehringer Ingelheim, grants from Gilead Sciences, Inc., grants from MSD K.K., and grants from Yakult Honsha Co. unrelated to the present study. Dr. Boku reports grants and personal fees from Ono Pharmaceutical Co., Ltd., grants and personal fees from Bristol-Myers Squibb, grants and personal fees from Taiho Pharmaceutical Co., Ltd., personal fees from Chugai Pharmaceutical Co., Ltd., and personal fees from Eli-Lilly Japan K.K. unrelated to the present study. Dr. Hyodo reports grants and personal fees from Chugai Pharmaceutical Co., Ltd., grants and personal fees from Taiho Pharmaceutical Co., Ltd., grants and personal fees from Eli Lilly Japan K.K., grants and personal fees from Daiichi Sankyo Co., Ltd., and grants and personal fees from Ono Pharmaceutical Co., Ltd., unrelated to the present study. Dr. Mizusawa reports grants from Ministry of Health, Labour and Welfare, Japan, grants from AMED, during the conduct of the study; personal fees from Chugai Pharmaceutical Co., Ltd., unrelated to the present study. Dr. Hara reports grants from AMED, during the conduct of the study; grants from Astra Zeneca K.K., grants and personal fees from Daiichi Sankyo Co., Ltd., grants from Sumitomo Dainippon Pharma Co., Ltd., personal fees from Eli Lilly Japan K.K., grants from Merck Serono Co., Ltd., grants and personal fees from MSD K.K., grants and personal fees from Taiho Pharmaceutical Co., Ltd., grants and personal fees from Chugai Pharmaceutical Co., Ltd., grants from Eisai Co., grants from LSK BioPharma, grants from Incyte, grants from Pfizer Inc., grants from Boehringer Ingelheim, grants from BeiGene, grants and personal fees from Ono Pharmaceutical Co., Ltd., grants and personal fees from Bristol-Myers Squibb, personal fees from Yakult Honsha Co., personal fees from Sanofi K.K., and personal fees from Takeda Pharmaceutical Co., Ltd., unrelated to the present study. Dr. Nishina reports grants and personal fees from Taiho Pharmaceutical Co., Ltd., grants and personal fees from Chugai Pharmaceutical Co., Ltd., grants from Daiichi Sankyo Co., Ltd., grants from MSD K.K., grants and personal fees from Ono Pharmaceutical Co., Ltd., grants and personal fees from Bristol-Myers Squibb, grants and personal fees from Eli Lilly Japan K.K., and grants from Sumitomo Dainippon Pharma Co., Ltd., unrelated to the present study. Dr. Sakamoto has nothing to disclose. Dr. Shitara reports grants and personal fees from Astellas Pharma Inc., grants and personal fees from Eli Lilly Japan K.K., personal fees from Bristol-Myers Squibb, personal fees from Takeda Pharmaceutical Co., Ltd., personal fees from Pfizer Inc., grants and personal fees from Ono Pharmaceutical Co., Ltd., personal fees from Novartis, personal fees from AbbVie, personal fees from Yakult Honsha, grants from Sumitomo Dainippon Pharma Co., Ltd., grants from Daiichi Sankyo Co., Ltd., grants from Taiho Pharmaceutical Co., Ltd., grants from Chugai Pharmaceutical Co., Ltd., grants and personal fees from MSD K.K., and grants from Medi-Science, Inc. unrelated to the present work. Dr. Shinozaki reports personal fees from Chugai Pharmaceutical Co., Ltd., personal fees from Takeda Pharmaceutical Co., Ltd., personal fees from Mochida Pharmaceutical Co., Ltd., personal fees from Merck Serono Co., Ltd., personal fees from Taiho Pharmaceutical Co., Ltd., personal fees from Yakult Honsha Co., personal fees from Ono Pharmaceutical Co., Ltd., personal fees from Eisai Co., personal fees from Shionogi & Co., personal fees from Eli Lilly Japan K.K., personal fees from Sanofi K.K., personal fees from Daiichi Sankyo Co., Ltd., personal fees from Bayer Yakuhin, Ltd., and personal fees from Pfizer Inc. unrelated to the present study. Dr. Katayama reports grants and nonfinancial support from the Ministry of Health, Labour and Welfare, Japan, grants and nonfinancial support from AMED, during the conduct of the study, and personal fees from Johnson & Johnson K.K. unrelated to the present study. Dr. Nakamura has nothing to disclose. Dr. Muro reports grants from Gilead Sciences, Inc., grants from Daiichi Sankyo Co., Ltd., grants from Shionogi & Co., grants from MSD K.K., grants from Sanofi K.K., grants from Kyowa Kirin Co., Ltd., grants from Merck Serono Co., Ltd., grants from Pfizer Inc., personal fees from Eli Lilly Japan K.K., personal fees from Chugai Pharmaceutical Co., Ltd., personal fees from Takeda Pharmaceutical Co., Ltd., personal fees from Taiho Pharmaceutical Co., Ltd., personal fees from Sanofi K.K., personal fees from Bayer Yakuhin, Ltd., and personal fees from Bristol-Myers Squibb unrelated to the present study. Dr. Terashima reports personal fees from Taiho Pharmaceutical Co., Ltd., personal fees from Chugai Pharmaceutical Co., Ltd., personal fees from Ono Pharmaceutical Co., Ltd., personal fees from Bristol-Myers Squibb, personal fees from Yakult Honsha, personal fees from Takeda Pharmaceutical Co., Ltd., personal fees from Eli Lilly Japan K.K., personal fees from Pfizer Inc., and personal fees from Daiichi Sankyo Co., Ltd., unrelated to the present study.
Ethical approval
The review committees of the JCOG and WJOG and the institutional review boards of all participating institutions approved the study protocol, which was conducted according to the Declaration of Helsinki and Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects.
Informed consent
Informed consent to be included in the study was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nakajima, T.E., Yamaguchi, K., Boku, N. et al. Randomized phase II/III study of 5-fluorouracil/l-leucovorin versus 5-fluorouracil/l-leucovorin plus paclitaxel administered to patients with severe peritoneal metastases of gastric cancer (JCOG1108/WJOG7312G). Gastric Cancer 23, 677–688 (2020). https://doi.org/10.1007/s10120-020-01043-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-020-01043-x