Abstract
Purpose
Fedratinib is an oral and selective Janus kinase 2 inhibitor that is indicated for treatment of adults with intermediate-2 or high-risk primary or secondary myelofibrosis. Fedratinib is metabolized by cytochrome P450s (CYPs), primarily CYP3A4. The objective of this study was to determine the effects of the strong CYP3A4 inducer rifampin and moderate CYP3A4 inducer efavirenz on the pharmacokinetics of single doses of fedratinib.
Methods
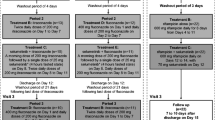
This Phase 1, open-label, two-part study (Part 1 for rifampin and Part 2 for efavirenz) was conducted in healthy adult men and women. A single dose of fedratinib (500 mg) was administered on Day 1. Participants received rifampin 600 mg daily or efavirenz 600 mg daily on Days 9–18. On Day 17, a single dose of fedratinib (500 mg) was coadministered with rifampin or efavirenz. Plasma fedratinib concentrations were measured using validated liquid chromatography–tandem mass spectrometry.
Results
Maximum observed plasma fedratinib concentrations were lowered by approximately 70% and 30% during coadministration with rifampin or efavirenz, respectively, compared with fedratinib alone. Geometric means of fedratinib area under the plasma concentration–time curve from 0 to infinity were decreased by 81% (90% confidence interval [CI], 77–83%) and 47% (90% CI, 40–53%) during coadministration with rifampin or efavirenz, respectively. Fedratinib was generally well tolerated when administered alone or in combination with rifampin or efavirenz.
Conclusion
Significant reductions in fedratinib exposure were observed in the presence of strong or moderate CYP3A4 inducers. These results suggest that agents that are strong or moderate inducers of CYP3A4 should be avoided when coadministered with fedratinib.
Trial Registration Number
NCT03983239 (Registration date: June 12, 2019).


Similar content being viewed by others
Data availability
Data requests may be submitted to Celgene, a Bristol Myers Squibb Company, at https://vivli.org/ourmember/celgene/ and must include a description of the research proposal.
References
Furqan M, Mukhi N, Lee B, Liu D (2013) Dysregulation of JAK-STAT pathway in hematological malignancies and JAK inhibitors for clinical application. Biomark Res 1(1):5. https://doi.org/10.1186/2050-7771-1-5
Schwartz DM, Bonelli M, Gadina M, O’Shea JJ (2016) Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol 12(1):25–36. https://doi.org/10.1038/nrrheum.2015.167
Vainchenker W, Constantinescu SN (2013) JAK/STAT signaling in hematological malignancies. Oncogene 32(21):2601–2613. https://doi.org/10.1038/onc.2012.347
Vainchenker W, Leroy E, Gilles L, Marty C, Plo I, Constantinescu SN (2018) JAK inhibitors for the treatment of myeloproliferative neoplasms and other disorders. F1000Res 7:82. https://doi.org/10.12688/f1000research.13167.1
(2019) Inrebic (fedratinib) product information. Celgene Corporation
Ogasawara K, Xu C, Kanamaluru V, Siebers N, Surapaneni S, Ridoux L, Palmisano M, Krishna G (2020) Excretion balance and pharmacokinetics following a single oral dose of [(14)C]-fedratinib in healthy subjects. Cancer Chemother Pharmacol 86(2):307–314. https://doi.org/10.1007/s00280-020-04121-0
Ogasawara K, Xu C, Kanamaluru V, Palmisano M, Krishna G (2020) Effects of repeated oral doses of ketoconazole on a sequential ascending single oral dose of fedratinib in healthy subjects. Cancer Chemother Pharmacol 85(5):899–906. https://doi.org/10.1007/s00280-020-04067-3
US Food and Drug Administration (2020) Clinical drug interaction studies—study design, data analysis, and clinical implications, guidance for industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions. Accessed 17 Aug 2020
US Food and Drug Administration (2020) Drug development and drug interactions: table of substrates, inhibitors and inducers. https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers#table3-3. Accessed 27 Aug 2020
Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivistö KT (2003) Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet 42(9):819–850. https://doi.org/10.2165/00003088-200342090-00003
Xu Y, Zhou Y, Hayashi M, Shou M, Skiles GL (2011) Simulation of clinical drug–drug interactions from hepatocyte CYP3A4 induction data and its potential utility in trial designs. Drug Metab Dispos 39(7):1139–1148. https://doi.org/10.1124/dmd.111.038067
Reitman ML, Chu X, Cai X, Yabut J, Venkatasubramanian R, Zajic S, Stone JA, Ding Y, Witter R, Gibson C, Roupe K, Evers R, Wagner JA, Stoch A (2011) Rifampin’s acute inhibitory and chronic inductive drug interactions: experimental and model-based approaches to drug–drug interaction trial design. Clin Pharmacol Ther 89(2):234–242. https://doi.org/10.1038/clpt.2010.271
Mouly S, Lown KS, Kornhauser D, Joseph JL, Fiske WD, Benedek IH, Watkins PB (2002) Hepatic but not intestinal CYP3A4 displays dose-dependent induction by efavirenz in humans. Clin Pharmacol Ther 72(1):1–9. https://doi.org/10.1067/mcp.2002.124519
Ogasawara K, Vince B, Xu C, Zhang M, Palmisano M, Krishna G (2020) A phase I study of the effect of repeated oral doses of pantoprazole on the pharmacokinetics of a single dose of fedratinib in healthy male subjects. Cancer Chemother Pharmacol 85(5):995–1001. https://doi.org/10.1007/s00280-020-04074-4
Ogasawara K, Zhou S, Krishna G, Palmisano M, Li Y (2019) Population pharmacokinetics of fedratinib in patients with myelofibrosis, polycythemia vera, and essential thrombocythemia. Cancer Chemother Pharmacol 84(4):891–898. https://doi.org/10.1007/s00280-019-03929-9
Ogasawara K, LoRusso PM, Olszanski AJ, Rixe O, Xu C, Yin J, Palmisano M, Krishna G (2020) Assessment of effects of repeated oral doses of fedratinib on inhibition of cytochrome P450 activities in patients with solid tumors using a cocktail approach. Cancer Chemother Pharmacol 86(1):87–95. https://doi.org/10.1007/s00280-020-04102-3
Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM, Silverman MH, Gilliland DG, Shorr J, Tefferi A (2011) Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol 29(7):789–796. https://doi.org/10.1200/JCO.2010.32.8021
Pardanani A, Tefferi A, Jamieson C, Gabrail NY, Lebedinsky C, Gao G, Liu F, Xu C, Cao H, Talpaz M (2015) A phase 2 randomized dose-ranging study of the JAK2-selective inhibitor fedratinib (SAR302503) in patients with myelofibrosis. Blood Cancer J 5(8):e335. https://doi.org/10.1038/bcj.2015.63
Wu F, Krishna G, Surapaneni S (2020) Physiologically based pharmacokinetic modeling to assess metabolic drug–drug interaction risks and inform the drug label for fedratinib. Cancer Chemother Pharmacol 86(4):461–473. https://doi.org/10.1007/s00280-020-04131-y
Wagner C, Pan Y, Hsu V, Sinha V, Zhao P (2016) Predicting the effect of CYP3A inducers on the pharmacokinetics of substrate drugs using physiologically based pharmacokinetic (PBPK) modeling: an analysis of PBPK submissions to the US FDA. Clin Pharmacokinet 55(4):475–483. https://doi.org/10.1007/s40262-015-0330-y
Elmeliegy M, Vourvahis M, Guo C, Wang DD (2020) Effect of P-glycoprotein (P-gp) inducers on exposure of P-gp substrates: review of clinical drug–drug interaction studies. Clin Pharmacokinet 59(6):699–714. https://doi.org/10.1007/s40262-020-00867-1
Takano J, Maeda K, Bolger MB, Sugiyama Y (2016) The prediction of the relative importance of CYP3A/P-glycoprotein to the nonlinear intestinal absorption of drugs by advanced compartmental absorption and transit model. Drug Metab Dispos 44(11):1808–1818. https://doi.org/10.1124/dmd.116.070011
Oswald S, Meyer zu Schwabedissen HE, Nassif A, Modess C, Desta Z, Ogburn ET, Mostertz J, Keiser M, Jia J, Hubeny A, Ulrich A, Runge D, Marinova M, Lötjohann D, Kroemer HK, Siegmund W (2012) Impact of efavirenz on intestinal metabolism and transport: insights from an interaction study with ezetimibe in healthy volunteers. Clin Pharmacol Ther 91(3):506–513. https://doi.org/10.1038/clpt.2011.255
Shi JG, Chen X, Emm T, Scherle PA, McGee RF, Lo Y, Landman RR, McKeever EG Jr, Punwani NG, Williams WV, Yeleswaram S (2012) The effect of CYP3A4 inhibition or induction on the pharmacokinetics and pharmacodynamics of orally administered ruxolitinib (INCB018424 phosphate) in healthy volunteers. J Clin Pharmacol 52(6):809–818. https://doi.org/10.1177/0091270011405663
Teo YL, Ho HK, Chan A (2015) Metabolism-related pharmacokinetic drug–drug interactions with tyrosine kinase inhibitors: current understanding, challenges and recommendations. Br J Clin Pharmacol 79(2):241–253. https://doi.org/10.1111/bcp.12496
Zhang M, Xu CR, Shamiyeh E, Liu F, Yin JY, von Moltke LL, Smith WB (2014) A randomized, placebo-controlled study of the pharmacokinetics, pharmacodynamics, and tolerability of the oral JAK2 inhibitor fedratinib (SAR302503) in healthy volunteers. J Clin Pharmacol 54(4):415–421. https://doi.org/10.1002/jcph.218
Harrison CN, Schaap N, Vannucchi AM, Kiladjian J-J, Jourdan E, Silver RT, Schouten HC, Passamonti F, Zweegman S, Talpaz M, Verstovsek S, Rose S, Shen J, Berry T, Brownstein C, Mesa RA (2020) Fedratinib in patients with myelofibrosis previously treated with ruxolitinib: an updated analysis of the JAKARTA2 study using stringent criteria for ruxolitinib failure. Am J Hematol 95(6):594–603. https://doi.org/10.1002/ajh.25777
Pardanani A, Harrison C, Cortes JE, Cervantes F, Mesa RA, Milligan D, Masszi T, Mishchenko E, Jourdan E, Vannucchi AM, Drummond MW, Jurgutis M, Kuliczkowski K, Gheorghita E, Passamonti F, Neumann F, Patki A, Gao G, Tefferi A (2015) Safety andand efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol 1(5):643–651. https://doi.org/10.1001/jamaoncol.2015.1590
Zhang M, Xu C, Ma L, Shamiyeh E, Yin J, von Moltke LL, Smith WB (2015) Effect of food on the bioavailability and tolerability of the JAK2-selective inhibitor fedratinib (SAR302503): results from two phase I studies in healthy volunteers. Clin Pharmacol Drug Dev 4(4):315–321. https://doi.org/10.1002/cpdd.161
Ogasawara K, Smith WB, Xu C, Yin J, Palmisano M, Krishna G (2020) Pharmacokinetics and tolerability of fedratinib, an oral, selective Janus kinase 2 inhibitor, in subjects with renal or hepatic impairment. Cancer Chemother Pharmacol 85(6):1109–1117. https://doi.org/10.1007/s00280-020-04084-2
Ogasawara K, Xu C, Yin J, Darpo B, Carayannopoulos L, Xue H, Palmisano M, Krishna G (2020) Evaluation of the potential for QTc prolongation with repeated oral doses of fedratinib in patients with advanced solid tumors. Clin Pharmacol Drug Dev. https://doi.org/10.1002/cpdd.1850.10.1002/cpdd.850
Acknowledgements
The authors thank study participants and their families who made this study possible and the clinical study teams who participated in the study. The study was supported by Bristol Myers Squibb. Medical writing support was provided by Bridget Sackey Aboagye, PhD, and Alex Loeb, PhD, of Chrysalis Medical Communications, Hamilton, NJ, and funded by Bristol Myers Squibb.
Funding
The clinical trial reported in this manuscript was designed and sponsored by Bristol Myers Squibb.
Author information
Authors and Affiliations
Contributions
KO and GK contributed to the study design, data analysis, interpretation, and draft of the manuscript. All authors critically reviewed the draft of the manuscript, approved the final version to be published, and agreed to be accountable for all aspects of the work. LNC, LL, and ML contributed to the study design, data analysis, and interpretation. MT contributed to the study design, data acquisition, and interpretation. JK and YX contributed to the data acquisition and interpretation. SS, SZ, and MP contributed to the study design and interpretation.
Corresponding author
Ethics declarations
Conflict of interest
KO, MT, LL, ML, YX, SS, LNC, SZ, MP, and GK are employees of and hold equity ownership in Bristol Myers Squibb. JK is an employee of Covance.
Research involving human participants and/or animals
All study procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent to participate was obtained from all individual participants included in the study.
Consent for publication
The authors consented to submit the manuscript for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ogasawara, K., Kam, J., Thomas, M. et al. Effects of strong and moderate CYP3A4 inducers on the pharmacokinetics of fedratinib in healthy adult participants. Cancer Chemother Pharmacol 88, 369–377 (2021). https://doi.org/10.1007/s00280-021-04292-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04292-4