Abstract
Purpose
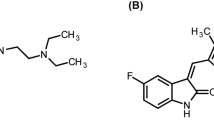
Fedratinib, an oral, selective Janus kinase 2 inhibitor with activity against both wild-type and mutant Janus kinase 2, has pH-dependent solubility, with free solubility at pH 1. Concomitant administration of drugs that reduce gastric acid secretion, such as pantoprazole, may decrease the absorption of fedratinib and affect patient outcomes. The aim of this study was to evaluate the impact of 7-day repeated 40-mg doses of pantoprazole on the pharmacokinetic (PK) properties of a single 500-mg dose of fedratinib in healthy male subjects.
Methods
In this phase I, single-center, open-label, two-period, two-treatment, fixed-sequence crossover study, healthy male subjects were administered a single dose of fedratinib 500 mg on day 1 in Period 1, followed by pantoprazole 40 mg daily for 7 days (day 1 to day 7) and a single dose of fedratinib 500 mg on day 7 in Period 2. After the discontinuation of nine subjects due to vomiting, the protocol was amended to provide ondansetron as antiemetic prophylaxis to an additional ten enrolled subjects.
Results
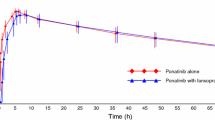
Twenty-six subjects were included. Repeated doses of pantoprazole 40 mg resulted in clinically insignificant increases in fedratinib exposure. Maximum plasma concentration increased by 1.09-fold and area under the plasma concentration–time curve from time 0 to infinity increased by 1.15-fold. All treatment-emergent adverse events were mild or moderate, except for one instance of neutropenia, which was considered unrelated to study intervention.
Conclusion
Coadministration with pantoprazole did not have clinically meaningful effects on fedratinib PK. No new or unexpected safety signals were observed with fedratinib.


Similar content being viewed by others
References
Kleppe M, Kwak M, Koppikar P, Riester M, Keller M, Bastian L, Hricik T, Bhagwat N, McKenney AS, Papalexi E, Abdel-Wahab O, Rampal R, Marubayashi S, Chen JJ, Romanet V, Fridman JS, Bromberg J, Teruya-Feldstein J, Murakami M, Radimerski T, Michor F, Fan R, Levine RL (2015) JAK-STAT pathway activation in malignant and nonmalignant cells contributes to MPN pathogenesis and therapeutic response. Cancer Discov 5(3):316–331. https://doi.org/10.1158/2159-8290.CD-14-0736
Inrebic (fedratinib) (package insert) (2019) Celgene Corporation, Summit
Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM, Silverman MH, Gilliland DG, Shorr J, Tefferi A (2011) Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol 29(7):789–796. https://doi.org/10.1200/JCO.2010.32.8021
Pardanani A, Tefferi A, Jamieson C, Gabrail NY, Lebedinsky C, Gao G, Liu F, Xu C, Cao H, Talpaz M (2015) A phase 2 randomized dose-ranging study of the JAK2-selective inhibitor fedratinib (SAR302503) in patients with myelofibrosis. Blood Cancer J 5:e335. https://doi.org/10.1038/bcj.2015.63
Pardanani A, Harrison C, Cortes JE, Cervantes F, Mesa RA, Milligan D, Masszi T, Mishchenko E, Jourdan E, Vannucchi AM, Drummond MW, Jurgutis M, Kuliczkowski K, Gheorghita E, Passamonti F, Neumann F, Patki A, Gao G, Tefferi A (2015) Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol 1(5):643–651. https://doi.org/10.1001/jamaoncol.2015.1590
U.S. Food and Drug Administration (2019) U.S. Food and Drug Administration. Drug approval package: INREBIC—product quality review(s). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212327Orig1s000ChemR.pdf. Accessed 26 Mar 2020.
Wolfe MM, Sachs G (2000) Acid suppression: optimizing therapy for gastroduodenal ulcer healing, gastroesophageal reflux disease, and stress-related erosive syndrome. Gastroenterology 118(2 Suppl 1):S9–31. https://doi.org/10.1016/s0016-5085(00)70004-7
Strand DS, Kim D, Peura DA (2017) 25 years of proton pump inhibitors: a comprehensive review. Gut Liver 11(1):27–37. https://doi.org/10.5009/gnl15502
Hagymasi K, Mullner K, Herszenyi L, Tulassay Z (2011) Update on the pharmacogenomics of proton pump inhibitors. Pharmacogenomics 12(6):873–888. https://doi.org/10.2217/pgs.11.4
Meyer UA (1996) Interaction of proton pump inhibitors with cytochromes P450: consequences for drug interactions. Yale J Biol Med 69(3):203–209
Zhang M, Xu CR, Shamiyeh E, Liu F, Yin JY, von Moltke LL, Smith WB (2014) A randomized, placebo-controlled study of the pharmacokinetics, pharmacodynamics, and tolerability of the oral JAK2 inhibitor fedratinib (SAR302503) in healthy volunteers. J Clin Pharmacol 54(4):415–421. https://doi.org/10.1002/jcph.218
Zhang M, Xu C, Ma L, Shamiyeh E, Yin J, von Moltke LL, Smith WB (2015) Effect of food on the bioavailability and tolerability of the JAK2-selective inhibitor fedratinib (SAR302503): results from two phase I studies in healthy volunteers. Clin Pharmacol Drug Dev 4(4):315–321. https://doi.org/10.1002/cpdd.161
Protonix (pantoprazole) (package insert) (2019) Pfizer Inc., Philadelphia.
Simon B, Muller P, Marinis E, Luhmann R, Huber R, Hartmann R, Wurst W (1990) Effect of repeated oral administration of BY 1023/SK&F 96022—a new substituted benzimidazole derivative—on pentagastrin-stimulated gastric acid secretion and pharmacokinetics in man. Aliment Pharmacol Ther 4(4):373–379. https://doi.org/10.1111/j.1365-2036.1990.tb00483.x
Ogasawara K, Zhou S, Krishna G, Palmisano M, Li Y (2019) Population pharmacokinetics of fedratinib in patients with myelofibrosis, polycythemia vera, and essential thrombocythemia. Cancer Chemother Pharmacol 84(4):891–898. https://doi.org/10.1007/s00280-019-03929-9
Burdick RK, Graybill, F.A. (1992) Confidence intervals on variance components, vol 127. In: Statistics, a series of textbooks and monographs. Marcel Dekker, New York
Lin L, Wong H (2017) Predicting oral drug absorption: mini review on physiologically-based pharmacokinetic models. Pharmaceutics 9(4). https://doi.org/10.3390/pharmaceutics9040041
Mudie DM, Amidon GL, Amidon GE (2010) Physiological parameters for oral delivery and in vitro testing. Mol Pharm 7(5):1388–1405. https://doi.org/10.1021/mp100149j
Lahner E, Annibale B, Delle Fave G (2009) Systematic review: impaired drug absorption related to the co-administration of antisecretory therapy. Aliment Pharmacol Ther 29(12):1219–1229. https://doi.org/10.1111/j.1365-2036.2009.03993.x
Ogawa R, Echizen H (2010) Drug-drug interaction profiles of proton pump inhibitors. Clin Pharmacokinet 49(8):509–533. https://doi.org/10.2165/11531320-000000000-00000
Niwa T, Yamamoto S, Saito M, Kobayashi N, Ikeda K, Noda Y, Takagi A (2006) Effects of serotonin-3 receptor antagonists on cytochrome P450 activities in human liver microsomes. Biol Pharm Bull 29(9):1931–1935. https://doi.org/10.1248/bpb.29.1931
Marciani L, Wright J, Foley S, Hoad CL, Totman JJ, Bush D, Hartley C, Armstrong A, Manby P, Blackshaw E, Perkins AC, Gowland PA, Spiller RC (2010) Effects of a 5-HT(3) antagonist, ondansetron, on fasting and postprandial small bowel water content assessed by magnetic resonance imaging. Aliment Pharmacol Ther 32(5):655–663. https://doi.org/10.1111/j.1365-2036.2010.04395.x
Funding
The clinical trial reported in this manuscript was sponsored by Sanofi. Medical writing support was provided by Shawn Vahabzadeh, PharmD, of MediTech Media and funded by Bristol Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KO, MP, and GK are employed by and hold equity ownership in Bristol Myers Squibb. CX and MZ are employed by and hold equity ownership in Sanofi. BV has nothing to disclose.
Ethical approval
All study procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual subjects included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ogasawara, K., Vince, B., Xu, C. et al. A phase I study of the effect of repeated oral doses of pantoprazole on the pharmacokinetics of a single dose of fedratinib in healthy male subjects. Cancer Chemother Pharmacol 85, 995–1001 (2020). https://doi.org/10.1007/s00280-020-04074-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04074-4