Abstract
Conspecific cues often provide social information on habitat quality that is considered when deciding to settle at a specific site. The type of sensory cues useful for this will depend on the environment. For amphisbaenians, reptiles adapted to an underground life with highly reduced sight, chemoreception is especially useful to recognize conspecifics. Here, we first analyzed the lipophilic compounds from precloacal gland secretions of the amphisbaenian Blanus cinereus, showing that there were sex- and size-related variations in the proportions of the three major compounds. Then, we tested in the laboratory whether there was an underground site selection based on conspecific chemical cues (substrate scent marks) in two different contexts. In loose substrates, both male and female amphisbaenians tended to choose first the scent-marked substrates more often when the individual that produced the scent, independently of its sex, was relatively larger than the focal individual. In contrast, inside semi-permanent galleries, males, but not females, chose the scent-marked gallery more often when the scent donor, independently of its sex, was relatively smaller. These results suggest that the proportions of compounds in scent marks may allow amphisbaenians to estimate the body size of the producer and that this information affects their site selection decisions. However, the different substrate-dependent responses suggest a different meaning and usefulness of scent marks depending on the context.
Significance statement
The presence of your conspecific in a site may indicate that this is a “quality” site to live in. If you are blind and live underground, smell is one of the best options for detecting conspecifics and assessing how good are your surroundings. Here, we test whether a blind amphisbaenian reptile that spends its life buried in sandy substrates uses conspecific chemical stimuli to choose where to settle. We found that this decision is influenced by the microhabitat type, sex, and the size difference between the individual that sniffs and the producer of the scent. Amphisbaenians seem to detect and assess conspecific traits based on the differences in compounds in their odors. Therefore, using conspecific scent to assess habitat quality may help fossorial animals to live underground.
Similar content being viewed by others
Introduction
Animals’ survival and reproductive success depend on their ability to locate necessary resources and avoid potentially detrimental situations. Hence, the evolutionary success of many taxa seems to be associated with the development of specific sensory modalities to locate resources and avoid risks (Stevens 2013). For instance, bats and cetaceans rely on echolocation for foraging and avoiding obstacles (Thomas et al. 2004), and color vision is the basis of mate choice in many birds and fishes (Endler et al. 2005). One important use of the sensory abilities of an animal occurs when deciding whether to select or avoid a habitat or a specific site to settle. In many species, conspecific cues are often used as “social or public information” about habitat quality (e.g., Stamps 1988; Farrell et al. 2012; reviewed in Danchin et al. 2004; Wagner and Danchin 2010; Buxton et al. 2020), and the detection and assessment of these cues are based on different types of sensory modes. Nevertheless, the evolution of sensory modalities is influenced by the restrictions imposed by the characteristics of each local environment (Wiley and Richards 1978; Alberts 1992b; Shine 2005; Bradbury and Vehrencamp 2011; Stevens 2013).
Chemoreception is a widespread sensory modality among vertebrates, and chemical stimuli are used for different purposes (Müller-Schwarze 2006; Wyatt 2014). For example, fathead minnows employ odors to attract females and recognize shoal mates (e.g., Cole and Smith 1992; Brown and Smith 1994), and some mammals use gland secretions or urine for conspecific discrimination (e.g., Swaisgood et al. 1999; Johnston 2003). In reptiles, chemoreception is essential for the different aspects of their biology (for a review see Mason and Parker 2010; Martín and López 2011). Many reptile species employ chemical cues for detecting prey (e.g., Cooper 1995; Recio et al. 2020) and predators (e.g., Amo et al. 2004; King et al. 2008) or for navigation (Chelazzi and Deflino 1986). Chemical cues also play a prominent role in intraspecific communication and reproduction of many reptiles (Martín and López 2011). In this regard, chemical signals allow for reptiles’ sex and familiar discrimination and self-recognition (Alberts 1992a; López et al. 2003; Gonzalo et al. 2004; Ibáñez et al. 2012). Furthermore, chemical signals may provide information about the characteristics of potential competitors (Greene et al. 2001; Martín and López 2007; Ibáñez et al. 2012) or potential mates (Martín and López 2000; Greene et al. 2001; O’Donnell et al. 2004). Finally, reptiles use chemical senses to follow conspecific scent trails for mating (LeMaster et al. 2001; Bull and Lindle 2002; Shine et al. 2005) or for locating overwintering hibernacula (Brown and MacLean 1983).
Amphisbaenians are a major distinctive group of reptiles, morphologically and functionally adapted to a fossorial life (Gans 1978). One of the adaptations for living underground is a reduced vision (Gans 1978). Hence, chemoreception may be particularly important for these fossorial reptiles (Cooper et al. 1994). In fact, several studies have shown that amphisbaenians use their vomeronasal system to detect odors of prey (López and Salvador 1992; Semhan et al. 2010; López et al. 2014), predators and potentially harmful species (López and Martín 1994, 2001), and different habitat chemical cues (López et al. 2002; Martín et al. 2021a). Some amphisbaenians have precloacal glands that produce holocrine secretions, especially during the breeding season, whose composition differs between sexes (López and Martín 2005, 2009). The microscopic morphology of these glands suggests that, as amphisbaenians move inside tunnels, the secretion plugs are abraded against the substrate releasing semiochemicals (Jared et al. 1999). These substrate scent marks might be important in intraspecific communication and home range recognition (Cooper et al. 1994; López et al. 2000). Moreover, behavioral studies show that some amphisbaenians are capable of short-range detection and discrimination between chemical cues of females and males (Cooper et al. 1994; López and Martín 2009), familiar and unfamiliar conspecifics (Martín et al. 2020, 2021b), or self-recognition (López et al. 1997; Martín et al. 2020). Together, these studies have brought to light the importance of chemoreception for the fossorial lifestyle of amphisbaenians. However, it remains unknown whether and to what extent chemical cues of amphisbaenians or other fossorial reptiles could be used as underground substrate scent marks. These scent marks might act as potential indicators of habitat quality, when individuals select a specific site, and might also be used to mark territories or to locate mates.
Here, we investigated whether chemical cues (substrate scent marks) from conspecifics affected underground site selection by the amphisbaenian Blanus cinereus. We first examined potential sex- and size-related variations in lipophilic chemical compounds from precloacal gland secretions of this species. Then, we designed two different laboratory approaches to test (i) whether adult individuals selected or avoided loose substrates or semi-permanent galleries with scents from conspecifics and (ii) whether the sex and size of the conspecifics affected the site selection. In previous studies, using tongue-flicks as the primary behavioral response suggesting chemical discrimination, it was shown that, in addition to conspecific discrimination, male B. cinereus were attracted to female odors but responded aggressively by biting cotton swabs bearing the scent of other males or specific compounds from precloacal secretions of males (Cooper et al. 1994; López et al. 1997; López and Martín 2009). Hence, we hypothesized that (i) males would select substrates and galleries with female odor while avoiding substrates scent-marked by other males. However, we also anticipated (ii) an effect of body size, with males avoiding substrates with the scent of relatively larger males, but not those with the scent of relatively smaller males. Also, given the relatively small amount of precloacal secretions produced by these animals, we expected that (iii) the potential effect of scent marks would be more pronounced inside semi-permanent galleries, where secretions would be deposited repeatedly over the same surfaces, than in loose substrates where secretion would be scattered and more randomly distributed, being difficult to detect and identify.
Methods
Study animals and maintenance
During March and April 2021, we captured under stones 25 adult male and 19 adult female B. cinereus amphisbaenians in an oak forest near Navacerrada (40°43′ N, 04°01′ W; Madrid, Spain). Animals were weighed (body mass: males: mean ± SE = 5.3 ± 0.3 g; females: 5.4 ± 0.4 g), and their snout-vent length (SVL) was measured (males: mean ± SE = 188 ± 3 mm; females: 189 ± 5 mm). After capture, we transported in the same day the amphisbaenians to “El Ventorrillo” MNCN-CSIC field station (5 km from the capture site), where they were kept individually in indoor terraria (40 × 30 × 30 cm) with a 10-cm depth substrate of loose coconut fiber. Amphisbaenians were fed mealworm pupae (Tenebrio molitor) three times a week. Amphisbaenians could attain an optimal body temperature by thigmothermy with the substrate (López et al. 1998), which was warmed using a heating cable placed below the terraria and connected to a thermostat set at 22 °C. This temperature was close to the substrate temperatures selected by B. cinereus in a thermal gradient (mean ± SE = 20.7 ± 0.5 °C , range = 17.8–23.6 °C; López et al. 1998). Water was provided daily by moistening the substrate with a water spray. Although amphisbaenians spent all the time buried in the fiber substrate, we kept a natural photoperiod with sunlight entering through two large windows.
To be able to monitor the location of buried amphisbaenians in the experiments without further disturbance, they had been individually marked with PIT tags (Biomark MiniHPT8; Biomark, Inc., Boise, ID, USA; length = 8.4 mm, diameter = 1.4 mm, weigh = 0.03 g) implanted subcutaneously in the upper right side of the body. This marking procedure has been tested in other amphisbaenian species, showing no long-term negative consequences for animals (Recio et al. 2019). At the end of the trials, all animals were released in an apparently healthy state to their field capture sites where PIT tag marks were useful for further population monitoring.
Chemical analyses of precloacal secretions
In the same study area, we captured additional individual amphisbaenians (10 males and 10 females) to harvest their precloacal gland secretions. Immediately after capture, we gently pressed around their precloacal pores with forceps to collect the secretion of pores directly in the glass vials with Teflon-lined stoppers. Vials were kept in an ice box during daily morning field work and in the midday stored in a freezer at −20 °C until being analyzed. During each of the sampling events, we also obtained blank control vials using the same procedure but without collecting secretion. These amphisbaenians were measured as indicated above and immediately released at their capture sites. We did not collect secretions from the individuals maintained in captivity to allow their secretions to scent-mark the substrates used in the behavioral tests (see below).
Samples of precloacal secretions were analyzed using gas chromatography coupled to mass spectrometry (Trace 2000 GC-MS, Finnigan-ThermoQuest). Analytical procedures were similar to those used in the previous studies (López and Martín 2005). Tentative identification of compounds was made by comparison of the mass spectra in the NIST/EPA/NIH 2002 library and using the information from previous descriptive studies where identifications were confirmed using standards (López and Martín 2005, 2009). We determined the relative proportion of the major compounds as the percentage of the total ion current by integrating the peak areas in the chromatogram using the Xcalibur software (Finnigan Co.). Before statistical comparisons, the relative areas were transformed following the formula Ln[(proportion)/(1 − proportion)], to correct the problem of non-independence between proportions (Aebischer et al. 1993; García-Roa et al. 2018). We used separated general linear models (GLMs) to test for differences in transformed relative proportions of the three major compounds found (dependent variable) between sexes (fixed factor) and in relation to body size (log10-transformed SVL; continuous factor) and including the interaction between sex and size in the models. When the interaction was significant, we calculated, separately for males and females, Pearson’s linear correlation coefficients between the proportion of the compound and body size.
Site selection tests
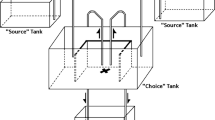
To test whether the presence of scents of conspecifics deposited on substrates affected the selection of novel sites by amphisbaenians, we considered two different situations that mimic natural conditions. In the field, this species selects preferentially sandy substrates in which amphisbaenians are not usually able to form semi-permanent galleries, as the loose sandy substrate often collapses immediately after an individual passes burrowing through it (Martín et al. 1991). However, semi-permanent galleries are observed in relatively harder (less sandy) substrates and under rocks that the species uses for thermoregulation and foraging (López et al. 1998; JM pers. observ.). Thus, Experiment 1 consisted of a choice test between two areas of loose substrates differing only in the fact that one of them had been used by conspecifics, presumably scent-marking this substrate with chemical cues, while the other area was a clean substrate. In contrast, in Experiment 2, we examined the underground selection of semi-permanent galleries. To simulate these galleries, we employed a T-maze approach with two arms (plastic tubes), one of them previously marked with conspecific odor.
In both experiments, amphisbaenians were tested with the scent of other individuals located at least 50 m from the responding individual, which, considering the low dispersal ability of amphisbaenians (Martín et al. 2021c) and because amphisbaenians were kept in separated individual cages in the laboratory, ensured that they had not had previous contact and could be considered unfamiliar. To minimize observer bias, blinded methods were used when all behavioral data were recorded and/or analyzed.
Experiment 1: loose substrate choice
Experiment 1 was conducted between the 20th and 30th of April with all the individuals captured and maintained in captivity (N = 44; 25 males, 19 females). We carried out experiments individually in testing plastic cages (71 × 46 × 37 cm) with their bottoms covered with a layer of clean loose substrate of coconut fiber about 5 cm deep. These testing cages were divided into two areas of equal surface (46 × 33 cm) with a 5-cm wide gap between them. In each of these areas, we added an additional amount of coconut fiber that differed in the chemicals it contained. On one of the halves of the cage (“control side”), randomly chosen, we added an additional amount of 200 g of clean coconut fiber. This clean fiber had been stored in the facilities where amphisbaenians were maintained, inside empty cages of the same type as those housing the amphisbaenians, and exposed to the same conditions of temperature and humidity. On the other half of the cage (“scent treatment side”), we added a substrate chosen among three treatments: (a) “Male,” (b) “Female,” or (c) “Control.” In “Male” and “Female” treatments, we added 200 g of coconut fiber taken from one home cage where another unfamiliar male or female conspecific had been kept individually for at least 2 weeks. In the “Control” treatment, we added 200 g of clean coconut fiber on the treatment side. All individuals were tested once per treatment on consecutive days (one treatment per day), following a randomized block design with a counterbalanced order of presentation. All trials were conducted during the morning (between 9 a.m. and 1 p.m., GTM) in the same room, illuminated with dim light and maintained at a temperature of 20 °C, close to the preferred temperature of this species (López et al. 1998).
Every test began by gently taking the focal individual from its home cage and placing it in the middle gap of a testing cage. Amphisbaenians explored the area for some seconds and quickly buried themselves in the substrate. Then, we noted every 15 min for 2 h (8 recordings) the position of the animal. Locating buried individuals without disturbance was possible by detecting the signal of its PIT tag (see above) using a hand-held portable reader (Biomark 601 Reader) placed above the substrate. Three behavioral variables were recorded for each individual: (a) the “First choice” (i.e., the location of the individual in the first measure, 15 min after being released), (b) the number of “Times” that the individual was recorded in the scent treatment side during all the trial, and (c) “Final choice,” only estimated for those individuals for which a visit to the scent treatment side was recorded at least once in each of the three trials (corresponding to the three treatments; N = 19; 10 males, 9 females). Regarding the “Final choice,” we considered that the scent treatment side of the cage was chosen when the proportion of times located in this half of the testing cage in relation to the total number of recordings after first visiting it was higher than expected by chance (probability of success = 0.5; tested using two-tailed binomial tests with binom.test function in the stats package; R Core Team 2022).
At the end of each test, amphisbaenians were returned to their home cages, and testing cages were cleaned with abundant water and left to dry for more than 12 h before the next trial, when new clean or scent-marked substrates were used.
Experiment 2: semi-permanent gallery choice
We carried out T-maze experiments between the 30th June and 2nd July with only part of the amphisbaenians maintained in captivity (15 males, body mass: mean ± SE = 4.9 ± 0.3 g, SVL: mean ± SE = 185 ± 5 mm; 14 females, body mass = 5.0 ± 0.4 g, SVL= 189 ± 5 mm). Underground semi-permanent galleries were simulated by using two transparent plastic tubes (length = 25 cm; internal diameter = 16 mm) joined by a T-shaped opaque plastic piece (length = 6 cm; width = 4 cm; internal diameter = 12 mm). Both tubes differed in the chemicals they contained. One of the tubes was always clean, while the other had been previously scent-marked with one of three possible treatments: “Male,” “Female,” or “Control.” Scents from “Male” or “Female” were obtained by leaving a conspecific male or female inside the tube for at least 12 h before each trial and removing it immediately before the tests. Each tube had a longitudinal fissure that allowed air to enter inside it and to prevent condensation. In the “Control” tests, both tubes were clean. The side location of the tubes in the T-maze was randomly chosen. We performed all trials in the same environmental conditions as in Experiment 1.
We started trials by gently taking an amphisbaenian from its home cage and releasing it on the substrate in front of the T-shaped plastic piece with the snout facing the entrance, so that the individual typically moved quickly inside the tubes. Then, we noted which arm (gallery) of the T-maze the amphisbaenian chose (variable “Initial choice”), i.e., the arm tube where the individual’s head and first third of the body trunk were first seen, after passing through the T-shaped piece. Then, amphisbaenians typically moved forward inside this arm tube. This was the only behavioral variable recorded in Experiment 2. At the end of each test, animals were returned to their home cages, and the tubes were cleaned with water as we did with the testing cages.
Statistical analyses of behavioral data
Modeling of behavioral data was conducted in the R statistical software (version 4.2.1; R Core Team 2022). We first made contingency tables with the observed number of individuals in each treatment that made an initial choice of the scent-marked side or arm and those that chose the control side or arm. Then, we performed two-tailed binomial tests with binom.test function in the stats package (R Core Team 2022) to test, for each scent treatment, whether the number of individuals that chose a particular side or arm was different than expected by chance (probability of success = 0.5). We also used Pearson’s chi-square tests for independence (chisq.test function in stats package; R Core Team 2022) to test for differences in side or arm choice among treatments.
In addition, we fitted generalized linear mixed models (GLMM) with glmer function in the lme4 R package (Bates et al. 2015) using a binomial distribution. In the models, we initially included the “Sex” of the focal individual and the “Scent” treatment (Male, Female, or Control) as fixed factors, the size (“SVL”) of the focal individual as a covariate, and the “Individual” as a random factor, according to our repeated measures design. We selected the model with the lowest Akaike value corrected for small sample sizes (AICc function in the MuMIn package; Barton 2020) and employed the likelihood ratio test (LRT) (lrtest function in the lmtest package; Zeileis and Hothorn 2002) as the omnibus test. After obtaining the most suitable model, we conducted Wald’s chi-square tests for mixed models (Anova function in the car package; Fox and Weisberg 2019) to estimate the effects of the covariate, the fixed factors, and their interactions.
In further analyses, we tested the effect of the “Size difference” between the focal responding individual and the one that had donated the scent (SVL focal − SVL donor; negative numbers indicating a relatively larger donor while positive numbers indicating a relatively small donor). We performed the same analyses as above but we replaced the covariate “SVL” with the covariate “Size difference.” Because in the “Control” scent treatments, there was no donor, and we excluded this treatment and analyzed only the trials with chemicals from another conspecific (i.e., “Male” and “Female”). Therefore, the order of stimulus presentation could not be considered as counterbalanced and, thus, to control for this effect, we nested the “Day” when each test was made within the “Individual” and included this as a random factor in the models for both experiments.
Results
Chemical variations of precloacal secretions
The compounds found in the precloacal secretions of amphisbaenians were similar to those found in the previous published analyses, with cholesteryl methyl ether (relative abundance, mean ± SE = 39.3 ± 3.0 %; range = 11.2–57.0 %) and cholesterol (35.8 ± 3.0 %; range = 22.6–70.2 %) being the most abundant compounds and with squalene also being relevant in some individuals (3.1 ± 1.0 %; range = 0.4–15.1 %). There were also some other minor compounds (less than 5 %) such as dodecanoic acid (3.7 %), cholesta-5,7-dien-3-ol acetate (3.3 %), or campesterol (1.5 %).
Relative proportions of cholesteryl methyl ether were significantly higher in males than in females (GLM, sex: F1.16 = 12.30, p = 0.003), and larger individuals had relatively lower proportions (SVL: F1.16 = 62.01, p < 0.0001), although the pattern of decrease in cholesteryl methyl ether with size differed slightly between sexes (interaction: F1.16 = 12.33, p = 0.003) (Pearson’s correlations, males: r = −0.92, p = 0.0009; females: r = −0.91, p = 0.0003) (Fig. 1a).
Relationships between relative proportions (% TIC area) of the three major compounds found in precloacal secretions (a cholesteryl methyl ether, b cholesterol, and c squalene) and body size (snout-to-vent length, SVL) of male (black dots, continuous line) and female (white dots, dashed line) B. cinereus amphisbaenians
Proportions of cholesterol were significantly higher in females than in males (GLM, sex: F1.16 = 14.82, p = 0.0014) and varied significantly with body size (SVL: F1.16 = 34.97, p < 0.0001) but with a different strength in each sex (interaction: F1.16 = 14.98, p = 0.0013), with cholesterol increasing significantly with size in females (Pearson’s correlation, r = 0.92, p = 0.0002), but not significantly in males (r = 0.47, p = 0.17) (Fig. 1b).
Finally, proportions of squalene were significantly higher in males than in females (GLM, sex: F1.16 = 9.72, p = 0.007) and varied significantly with body size (SVL: F1.16 = 5.92, p = 0.027) but in a different way in each sex (interaction: F1.16 = 10.44, p = 0.005), with squalene increasing significantly with size in males (Pearson’s correlation, r = 0.75, p = 0.012) but tending to decrease with size, although not significantly, in females (r = −0.36, p = 0.30) (Fig. 1c).
Experiment 1:loose substrate choice
In all scent treatments, amphisbaenians did not significantly select the control or the scent-marked side in their “First choice” more often than expected by chance (two-tailed binomial tests, focal males, Control: p = 0.11; Female scent: p = 0.42; Male scent: p = 0.23; focal females, Control: p = 0.36; Female scent: p = 0.36; Male scent: p = 0.99). Moreover, there were no significant differences among the scent treatments in the number of individuals that selected as their “First choice” the scent-marked side in a loose substrate, in comparison with an expected random selection of the sides, neither in male (Pearson’s chi-square test of independence, χ22 = 5.59, p = 0.06) nor female amphisbaenians (χ22 = 1.29, p = 0.52) (Fig. 2a). A similar lack of effect of the scent treatment was found when the “Final choice” was considered (males: χ22 = 0.36, p = 0.84; females: χ22 = 1.50, p = 0.47).
Results of the GLMMs for the choice of a side of the cage in a loose substrate showed that none of the selected models using the Akaike criterion successfully achieved statistical significance in the omnibus tests, except in the case of the “First choice” variable when the “Size difference” between the donor of scent and the focal animal and the “Scent” treatment were included in the model (LRT, χ22 = 12.49, p < 0.01) (Table S1). In this model, only the effect of the “Size difference” was significant (Wald’s test, χ21= 7.73, p = 0.0054), but the effect of “Scent” was not (Wald’s test, χ21= 2.40, p = 0.12). Therefore, both focal male and female amphisbaenians tended to choose first the scent-marked side more often when the donor individual that produced the scent was relatively larger than the focal individual, independently of the sex of the donor (Fig. 3).
Experiment 2: semi-permanent gallery choice
In all scent treatments, amphisbaenians did not significantly select the control or the scent-marked arm of a gallery in their “First choice” more often than expected by chance (two-tailed binomial tests, focal males, Control: p = 0.61; Female scent: p = 0.99; Male scent: p = 0.12; focal females, Control: p = 0.42; Female scent: p = 0.79; Male scent: p = 0.79). In addition, there were no significant differences between the scent treatments in the number of individuals that selected as their “First choice” the scent-marked arm inside a gallery, in comparison with an expected random selection of the arms, neither in male (Pearson’s chi-square test of independence, χ22 = 3.42, p = 0.18) nor female amphisbaenians (χ22 = 1.35, p = 0.51) (Fig. 2b).
When running GLMM models for the “First choice” of an arm inside a gallery, with “SVL” as a covariate, the model containing “Scent” seemed the most suitable according to the Akaike criterion, but the likelihood ratio test did not reach a significance (see Table S2). However, when including “Size difference” as a covariate instead of “SVL,” the model containing both “Sex” of the focal individual, “Scent” treatment, “Size difference,” and their interactions was the most suitable and reached a significance (LRT, χ27 = 21.70, p < 0.01) (Table S2). However, in this model, only the interaction between “Sex” and “Size difference” was significant (Wald’s test, χ21= 4.02, p = 0.045), while the rest of the factors and interactions did not reach a statistical significance (Wald’s tests, χ21 < 3.46, p > 0.063 in all cases). We ran further the GLMM analyses separately for males and females that included “Size difference” as a covariate, “Scent” treatment as a factor, and the “Day” nested within “Individual” as random factors. For males, we found that the model including “Size difference” had the lowest Akaike value (AICc null model = 46.35; AICc selected model = 41.22; AICc other models >> 43.64), and the omnibus test was statistically significant (LRT, χ21 = 7.81, p = 0.0052), although the chi-square test for the “Size difference” effect did not reach significance (Wald’s tests, χ21= 2.49, p = 0.11). For females, the model containing both “Scent” treatment and “Size difference” and their interaction was the most suitable under our criteria (AICc null model = 45.51; AICc selected model = 44.88; AICc other models >> 47.56; LRT, χ21 = 9.64, p = 0.022), but none of the factor was significant in the chi-square tests (Wald’s tests, “Scent,” χ21= 0.064, p = 0.80; “Size difference,” χ21= 0.016, p = 0.90; “Scent” × “Size difference,” χ21= 2.70, p = 0.10). Therefore, males tended to choose the scent-marked gallery more often when the donor of the scent was relatively smaller than them, independently of the sex of the scent donor, while a lack of effect was found for females (Fig. 4).
First choice of the control or scent-marked arm in semi-permanent galleries (Experiment 2) by focal a male or b female B. cinereus amphisbaenians in relation to body size difference (i.e., the SVL of the focal individual minus the SVL of the donor; negative numbers indicating a relatively larger donor) in treatments with scent of conspecific males or females
Discussion
This study found some weak, but significant, effects of substrate scent marks from conspecific on the underground site selection decisions in the amphisbaenians B. cinereus. These effects may be related to the observed inter-individual variability in compounds of the precloacal gland secretions from which scent marks very probably originated. However, the importance and direction of these effects seemed to be independent of the sex of the producer of the scent mark. Rather, they depended on the sex of the responding animal and on the microhabitat context (loose substrates vs. semi-permanent galleries), which would presumably affect the usefulness and meaning of scent marks.
Our chemical analyses first confirmed that the major compounds found in precloacal secretions of B. cinereus amphisbaenians clearly differed between sexes (see also López and Martín 2005, 2009). Detecting these chemical differences very likely allows amphisbaenians to discriminate between male and female scents, as it was found in previous experiments that measured tongue-flicking chemosensory responses (Cooper et al. 1994; López and Martín 2009). Based on these previous findings, we expected that, in the current experiments, amphisbaenians would have also shown differential responses to scent marks of males and females. However, we did not find that the sexual identity of the producer of the scent mark affected to the site or gallery selection decisions, neither in male nor female responses. This lack of effect might be simply explained if substrate scent marks would change their chemical characteristics after some time of being deposited, for example, because of chemical transformations of the compounds by oxidation due to the humidity of the substrate (Regnier and Goodwin 1977; Alberts 1992b; Apps et al. 2015) or by soil microorganisms (Murphy et al. 2007). In previous tongue-flick trials, amphisbaenians were able to discriminate the sexes when the fresh secretion was collected from a conspecific and immediately offered to the responding individual just in front of its snout (Cooper et al. 1994). However, compounds of scent marks in underground substrates might be altered, or the scent mark might be scattered and not be easily detected by the focal animals, which would preclude the scent mark to provide enough information to discriminate between sexes. Alternatively, the sexual identity of the producer might not be important if the mere detection of a scent mark of any conspecific was enough information to indicate the quality of a given site.
Our chemical analyses also showed the novel findings that there were clear relationships between the proportions of some major compounds in precloacal secretions of B. cinereus amphisbaenians and the body size of the producer. The behavioral experiments of site selection showed the effects of the difference in body size between the focal amphisbaenian and the producer of the scent-mark, suggesting that the proportion of compounds in scent marks may allow amphisbaenians to estimate the body size of the producer. For example, a large individual would have secretions with less cholesteryl methyl ether and more cholesterol and, in the case of males, also with more squalene. However, the size-related responses of amphisbaenians to scent marks were different inside the simulated semi-permanent galleries and in loose substrates. As predicted, it is likely that, in a loose substrate, the concentration of the secretion compounds that produces the scent was low, and the scent marks might be scattered, mixed, and dispersed over a large volume of substrate. This would occur because the conspecific scent donor would not be able to scent-mark repeatedly the same locations, as on each occasion it passed through different sections of the substrate. In that situation, in our experiment with loose substrates, the responding individuals might not always be aware of the presence of these scent marks or might not be able to identify them. This would explain why the responses observed in Experiment 1 (loose substrates) were similar in males and females and in most cases not significantly different. In contrast, in Experiment 2 (inside semi-permanent galleries), the scent marks would be more concentrated and more clearly defined, being more easily detected by the focal individuals and providing more information about the producer and thus allowing the more specific sex-related responses observed.
Moreover, the directions of the responses were also different depending on the microhabitat context. In loose substrates, amphisbaenians tended to select first the sites with scents of relatively larger animals. This might be explained if amphisbaenians selected these sites simply because the chemical size-related characteristics in secretions of these larger individuals (e.g., with more cholesterol or more squalene) might allow scent marks to be more easily detected in a loose substrate, while the scent of smaller individuals would not be easily detected, possibly leading to random responses. Therefore, these results might suggest that the mere detection of scent from any conspecific would indicate the “quality” of a new site, which would provide quick information for making a first choice decision about whether to continue exploring a new area. Similarly, many studies of crustaceans (Zimmer-Faust 1985), insects (Norris 1970), fish (Bett and Hinch 2015; Galbraith et al. 2017), amphibians (Aragón et al. 2000a; Secondi et al. 2005; Gautier et al. 2006), and reptiles (Aragón et al. 2001, 2006; Scott et al. 2013) demonstrated an attraction for conspecific scents when selecting a habitat (see reviews in Mason and Parker 2010; Buxton et al. 2020).
In contrast, inside galleries, where freshly and unaltered scent marks should be more evident and informative indicating the current presence of a conspecific, male amphisbaenians, but not females, avoided using galleries scent-marked by relatively larger individuals. This suggests that, in this context, male amphisbaenians were able to assess and consider not only the presence but also the competitive ability of the donor as indicated by the body size of the producer of the scent mark, very likely again based on the chemical characteristics of the secretions. The cost of encountering the individual that has produced the scent mark would depend on the competitive ability of both the signaler and the receiver (Gosling et al. 1996a, b). Although the existence of male combat in amphisbaenians has not been examined, previous experiments showed that males often respond aggressively to the scent of other males (and also to high concentrations of squalene alone, which is typical of secretion of males), but not to the scent of females (Cooper et al. 1994; López et al. 1997; López and Martín 2009), suggesting that male intrasexual aggression may exist. Thus, male amphisbaenians would avoid galleries used in the immediate past by a relatively larger resident individual because this could behave aggressively defending its “territory” in case of an encounter. In contrast, a relatively smaller conspecific could be easily defeated. Similar size-dependent responses to scent marks of conspecifics have been found in other species when assessing the quality of an unknown territory (Gosling et al. 1996a, b; Aragón et al. 2000b, 2001; Ibáñez et al. 2012).
Although this amphisbaenian species prefers sandy substrates, which are easier to dig (Martín et al. 1991), it also uses semi-permanent galleries formed under stones and in their close surroundings, which allows a quick and little costly access to these stones for thermoregulation and foraging (López et al. 1998). Our study suggests that scent marks might not be very useful in loose substrates but still sometimes may provide some approximate first guidance information on site quality. In contrast, scent marks inside galleries could have a more important and direct role in intraspecific communication and spatial orientation, also affecting settlement decisions. Similarly, other fossorial animals that use galleries to move underground, such as rodents, ants, or termites, use scent marks deposited inside these galleries for intraspecific communication (e.g., Vander Meer et al. 1998; Johnston 2003).
The weak and sometimes little specific responses to scent marks observed in this study might be explained if our experimental conditions confronting amphisbaenians with entirely new sites were not replicating a common situation in nature for these animals. Other species of amphisbaenians seem to show high site fidelity, with very short displacements around the same small areas (Martín et al. 2021c), which also seems to be the case in B. cinereus (JM unpubl. data). Therefore, the usefulness of scent marks of unknown conspecifics in the underground environment indicating habitat quality of new sites might also be limited if this amphisbaenians species is usually restricted to the same small areas where the conspecifics they can find are mostly familiar ones. Nevertheless, the responses to scent marks could be different depending on the familiarity or genetic relatedness between individuals, as other amphisbaenian species seem able of familiar chemosensory recognition (Martín et al. 2020, 2021b). Thus, in the fossorial environment, short-distance direct chemical communication between amphisbaenians, as shown on previous studies (e.g., Cooper et al. 1994), would be more important than scent marks. Nevertheless, we conclude that some information derived from conspecific scent marks still seems to be useful when selecting an underground new site. In loose substrates, disperse conspecific scents might simply indicate the “quality” of a new site. In contrast, inside galleries, more evident scent marks might indicate the current presence of an unfamiliar conspecific, which would force male amphisbaenians to also consider the competitive ability (body size) of the producer, avoiding galleries used by relatively larger individuals.
Data availability
Our data are available in Figshare at: https://doi.org/10.6084/m9.figshare.21583935.
References
Aebischer NJ, Robertson PA, Kenward RE (1993) Compositional analysis of habitat use from animal radio-tracking data. Ecology 74:1313–1325
Alberts AC (1992a) Pheromonal self-recognition in desert iguanas. Copeia 1992:229–232
Alberts AC (1992b) Constraints on the design of chemical communication systems in terrestrial vertebrates. Am Nat 139:62–89
Amo L, López P, Martı́n J (2004) Wall lizards combine chemical and visual cues of ambush snake predators to avoid overestimating risk inside refuges. Anim Behav 67:647–653
Apps PJ, Weldon PJ, Kramer M (2015) Chemical signals in terrestrial vertebrates: search for design features. Nat Prod Rep 32:1131–1153
Aragón P, López P, Martín J (2000) Conspecific chemical cues influence pond selection by male newts Triturus boscai. Copeia 2000:874–878
Aragón P, López P, Martín J (2001) Effects of conspecific chemical cues on settlement and retreat-site selection of male lizards, Lacerta monticola. J Herpetol 35:681–684
Aragón P, Massot M, Gasparini J, Clobert J (2006) Socially acquired information from chemical cues in the common lizard, Lacerta vivipara. Anim Behav 72:965–974
Barton K (2020) MuMIn: multi-model inference. R package version 1(43):17 https://CRAN.R-project.org/package=MuMIn
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed effects models using lme4. J Stat Softw 67:1–48
Bett NN, Hinch SG (2015) Attraction of migrating adult sockeye salmon to conspecifics in the absence of natal chemical cues. Behav Ecol 26:1180–1187
Bradbury J, Vehrencamp S (2011) Principles of animal communication, 2nd edn. Sinauer Associates, Sunderland, MA
Brown WS, MacLean FM (1983) Conspecific scent-trailing by newborn timber rattlesnakes, Crotalus horridus. Herpetologica 39:430–436
Brown GE, Smith RJF (1994) Fathead minnows use chemical cues to discriminate natural shoalmates from unfamiliar conspecifics. J Chem Ecol 20:3051–3061
Bull MC, Lindle C (2002) Following trails of partners in the monogamous lizard, Tiliqua rugosa. Acta Ethol 5:25–28
Buxton VL, Enos JK, Sperry JH, Ward MP (2020) A review of conspecific attraction for habitat selection across taxa. Ecol Evol 10:12690–12699
Chelazzi G, Delfino G (1986) A field test on the use of olfaction in homing by Testudo hermanni (Reptilia: Testudinidae). J Herpetol 20:451–455
Cole KS, Smith RJF (1992) Attraction of female fathead minnows, Pimephales promelas, to chemical stimuli from breeding males. J Chem Ecol 18:1269–1284
Cooper WE Jr (1995) Foraging mode, prey chemical discrimination, and phylogeny in lizards. Anim Behav 50:973–985
Cooper WE Jr, López P, Salvador A (1994) Pheromone detection by an amphisbaenı́an. Anim Behav 47:1401–1411
Danchin É, Giraldeau LA, Valone TJ, Wagner RH (2004) Public information: from nosy neighbors to cultural evolution. Science 305:487–491
Endler JA, Westcott DA, Madden JR, Robson T (2005) Animal visual systems and the evolution of color patterns: sensory processing illuminates signal evolution. Evolution 59:1795–1818
Farrell SL, Morrison ML, Campomizzi AJ, Wilkins RN (2012) Conspecific cues and breeding habitat selection in an endangered woodland warbler. J Anim Ecol 81:1056–1064
Fox J, Weisberg S (2019) An {R} companion to applied regression, 3rd edn. Sage Publications, Thousand Oaks
Galbraith HS, Blakeslee CJ, Schmucker AK, Johnson NS, Hansen MJ, Li W (2017) Donor life stage influences juvenile American eel Anguilla rostrata attraction to conspecific chemical cues. J Fish Biol 90:384–395
Gans C (1978) The characteristics and affinities of the Amphisbaenia. Trans Zool Soc Lond 34:347–416
García-Roa R, Saiz J, Gómara B, López P, Martín J (2018) How to tackle chemical communication? Relative proportions versus semiquantitative determination of compounds in lizard chemical secretions. Ecol Evol 8:2032–2040
Gautier P, Olgun K, Uzum N, Miaud C (2006) Gregarious behaviour in a salamander: attraction to conspecific chemical cues in burrow choice. Behav Ecol Sociobiol 59:836–841
Gonzalo A, Cabido C, Martín J, López P (2004) Detection and discrimination of conspecific scents by the anguid slow-worm Anguis fragilis. J Chem Ecol 30:1565–1573
Gosling LM, Atkinson NW, Collins SA, Roberts RJ, Walters RL (1996a) Avoidance of scent-marked areas depends on the intruder’s body size. Behaviour 133:491–502
Gosling LM, Atkinson NW, Dunn S, Collins SA (1996b) The response of subordinate male mice to scent marks varies in relation to their own competitive ability. Anim Behav 52:1185–1191
Greene MJ, Stark SL, Mason RT (2001) Pheromone trailing behavior of the brown tree snake, Boiga irregularis. J Chem Ecol 27:2193–2201
Ibáñez A, Marzal A, López P, Martín J (2013) Boldness and body size of male Spanish terrapins affect their responses to chemical cues of familiar and unfamiliar males. Behav Ecol Sociobiol 67:541–548
Jared C, Antoniazzi MM, Silva JRMC, Freymüller E (1999) Epidermal glands in Squamata: microscopical examination of precloacal glands in Amphisbaena alba (Amphisbaenia, Amphisbaenidae). J Morphol 241:197–206
Johnston RE (2003) Chemical communication in rodents: from pheromones to individual recognition. J Mammal 84:1141–1162
King R, Gosnell R, Mathis A (2008) Discrimination of predatory versus nonpredatory mammals by box turtles, Terrapene carolina. Chemoecol 18:61–64
LeMaster MP, Moore IT, Mason RT (2001) Conspecific trailing behaviour of red-sided garter snakes, Thamnophis sirtalis parietalis, in the natural environment. Anim Behav 61:827–833
López P, Martín J (1994) Responses by the amphisbaenian Blanus cinereus to chemicals from prey or potentially harmful ant species. J Chem Ecol 20:1113–1119
López P, Salvador A (1992) The role of chemosensory cues in discrimination of prey odors by the amphisbaenian Blanus cinereus. J Chem Ecol 18:87–93
López P, Martı́n J (2001) Chemosensory predator recognition induces specific defensive behaviours in a fossorial amphisbaenian. Anim Behav 62:259–264
López P, Martı́n J (2005) Intersexual differences in chemical composition of precloacal gland secretions of the amphisbaenian Blanus cinereus. J Chem Ecol 31: 2913–2921
López P, Martı́n J (2009) Potential chemosignals associated with male identity in the amphisbaenian Blanus cinereus. Chem Senses 34:479–486
López P, Salvador A, Cooper WE Jr (1997) Discrimination of self from other males by chemosensory cues in the amphisbaenian (Blanus cinereus). J Comp Psychol 111:105–109
López P, Salvador A, Martín J (1998) Soil temperatures, rock selection and the thermal ecology of the amphisbaenian reptile Blanus cinereus. Can J Zool 76:673–679
López P, Martín J, Barbosa A (2000) Site familiarity affects antipredator behavior of the amphisbaenian Blanus cinereus. Can J Zool 78:2142–2146
López P, Martín J, Cooper WE (2002) Chemosensory responses to plant chemicals by the amphisbaenian Blanus cinereus. Amphibia-Reptilia 23:348–353
López P, Martín J, Cuadrado M (2003) Chemosensory cues allow male lizards Psammodromus algirus to override visual concealment of sexual identity by satellite males. Behav Ecol Sociobiol 54:218–224
López P, Ortega J, Martín J (2014) Chemosensory prey detection by the amphisbaenian Trogonophis wiegmanni. J Herpetol 48:514–517
Martin J, López P (2000) Chemoreception, symmetry and mate choice in lizards. Proc R Soc Lond B 267:1265–1269
Martín J, López P (2007) Scent may signal fighting ability in male Iberian rock lizards. Biol Lett 3:125–127
Martín J, López P (2011) Pheromones and reproduction in reptiles. In: Norris DO, Lopez KH (eds) Hormones and reproduction of Vertebrates, Reptiles, Academic Press, vol 3. San Diego, CA, pp 141–167
Martín J, López P, Salvador A (1991) Microhabitat selection of the amphisbaenian Blanus cinereus. Copeia 1991:1142–1146
Martín J, Raya-García E, Ortega J, López P (2020) How to maintain underground social relationships? Chemosensory sex, partner and self recognition in a fossorial amphisbaenian. PLoS ONE 15:e0237188
Martín J, Ibáñez A, Garrido M, Raya-García E, López P (2021a) Chemical cues may allow a fossorial amphisbaenian reptile to avoid extremely saline soils when selecting microhabitats. J Arid Environ 188:104452
Martín J, Ortega J, García-Roa R, Jiménez-Robles O, Rodríguez-Ruiz G, Recio P, Cuervo JJ (2021c) Going underground: short- and long-term movements may reveal the fossorial spatial ecology of an amphisbaenian. Mov Ecol 9:14
Martín J, Raya-García E, Ortega J, López P (2021b) Offspring and adult chemosensory recognition by an amphisbaenian reptile may allow maintaining familiar links in the fossorial environment. PeerJ 9:e10780
Mason RT, Parker MR (2010) Social behavior and pheromonal communication in reptiles. J Comp Physiol A 196:729–749
Müller-Schwarze D (2006) Chemical ecology of vertebrates. Cambridge University Press, Cambridge
Murphy DV, Stockdale EA, Brookes PC, Goulding KW (2007) Impact of microorganisms on chemical transformations in soil. In: Abbott LK, Murphy DV (eds) Soil biological fertility. Springer, Dordrecht, pp 37–59
Norris MJ (1970) Aggregation response in ovipositing females of the desert locust, with special reference to the chemical factor. J Insect Physiol 16:1493–1515
O'Donnell RP, Ford NB, Shine R, Mason RT (2004) Male red-sided garter snakes, Thamnophis sirtalis parietalis, determine female mating status from pheromone trails. Anim Behav 68:677–683
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.r-project.org/. Accessed 30 Sep 2022
Recio P, Rodríguez-Ruiz G, Ortega J, Martín J (2019) PIT-Tags as a technique for marking fossorial reptiles: insights from a long-term field study of the amphisbaenian Trogonophis wiegmanni. Acta Herpetol 14:101–107
Recio P, Rodríguez-Ruiz G, Martín J (2020) Effects of sensory mode in prey discrimination and predatory behaviour of rock lizards. Amphibia-Reptilia 42:125–132
Regnier FE, Goodwin M (1977) On the chemical and environmental modulation of pheromone release from vertebrate scent marks. In: Müller-Schwarze D, Mozell MM (eds) Chemical Signals in Vertebrates. Plenum Press, London, pp 115–149
Scott ML, Whiting MJ, Webb JK, Shine R (2013) Chemosensory discrimination of social cues mediates space use in snakes, Cryptophis nigrescens (Elapidae). Anim Behav 85:1493–1500
Secondi J, Haerty W, Lode T (2005) Female attraction to conspecific chemical cues in the palmate newt Triturus helveticus. Ethology 111:726–735
Semhan RV, Halloy M, Montero R (2010) Chemical prey discrimination of termites in Amphisbaena heterozonata (Reptilia: Squamata): a learned trait? J Herpetol 44:489–492
Shine R (2005) All at sea: aquatic life modifies mate-recognition modalities in sea snakes (Emydocephalus annulatus, Hydrophiidae). Behav Ecol Sociobiol 57:591–598
Shine R, Webb JK, Lane A, Mason RT (2005) Mate location tactics in garter snakes: effects of rival males, interrupted trails and non-pheromonal cues. Funct Ecol 19:1017–1024
Stamps JA (1988) Conspecific attraction and aggregation in territorial species. Am Nat 131:329–347
Stevens M (2013) Sensory ecology, behaviour, and evolution. Oxford University Press, Oxford
Swaisgood RR, Lindburg DG, Zhou X (1999) Giant pandas discriminate individual differences in conspecific scent. Anim Behav 57:1045–1053
Thomas JA, Moss CF, Vater M (2004) Echolocation in bats and dolphins. University Chicago Press, Chicago
Vander Meer RK, Breed MD, Espelie KE, Winston ML (1998) Pheromone communication in social insects. CRC Press, London
Wagner RH, Danchin É (2010) A taxonomy of biological information. Oikos 119:203–209
Wiley RH, Richards DG (1978) Physical constraints on acoustic communication in the atmosphere: implications for the evolution of animal vocalizations. Behav Ecol Sociobiol 3:69–94
Wyatt TD (2014) Pheromones and animal behaviour. Chemical signals and signatures Cambridge University Press, Cambridge
Zeileis A, Hothorn T (2002) Diagnostic checking in regression relationships. R News 2:7–10
Zimmer-Faust RK (1985) Chemical attraction causing aggregation in the spiny lobster, Panulirus interruptus, and its probable ecological significance. Biol Bull 169:106–118
Acknowledgements
We thank two anonymous reviewers for their helpful comments and “El Ventorrillo” MNCN-CSIC Field Station for the use of their facilities.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was funded by Spanish Ministerio de Ciencia, Universidades e Innovación project PGC2018-093592-B-I00 (MCIU/AEI/FEDER, UE) and Ministerio de Ciencia e Innovación project PID2021-122358NB-I00 (MCIN/AEI /10.13039/501100011033 and ERDF A way of making Europe).
Author information
Authors and Affiliations
Contributions
All authors designed the methodology, collected and analyzed the data, contributed critically to the draft, and gave their final approval for the publication.
Corresponding author
Ethics declarations
Ethics approval
The captures enforced all the present Spanish laws and were performed under license granted by the “Dirección General de Biodiversidad y Recursos Naturales,” Comunidad Autónoma de Madrid (Spain) (Ref. 10/170740.9/21). The experiment followed ASAB (2020) guidelines for the ethical treatment of animals in behavioral research and was in accordance with the national animal welfare standards and protocols supervised by the “Comisión Ética de Experimentación Animal (CEEA)” of the Museo Nacional de Ciencias Naturales, CSIC, and the “Comité de Ética” of the Spanish National Research Council (CSIC) (Code:901/2020).
Competing interest
The authors declare no competing interests.
Additional information
Communicated by T. Madsen
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary materials 1:
Table S1 Results of the GLMMs performed for the choice of loose substrates (Experiment 1) by B. cinereus amphisbaenians. The complexity-AICc value criterion was used to select the models (analyses were replicated including the covariate ‘SVL’ or replacing it with the covariate ‘Size difference’; see Methods). Selected models are shown in bold. Likelihood ratio test (LRT) comparisons were made between the null model and the model with the lowest Akaike value. Table S2 Results of the GLMMs performed for the first choice of semi-permanent galleries (Experiment 2) by B. cinereus amphisbaenians. The complexity-AICc value criterion was used to select the models (analyses were replicated including the covariate ‘SVL’ or replacing it with the covariate ‘Size difference’; see Methods). Selected models are shown in bold. Likelihood ratio test (LRT) comparisons were made between the null model and the model with the lowest Akaike value.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Recio, P., Rodríguez-Ruiz, G., Sannolo, M. et al. Conspecific scent marks may influence underground site selection by a fossorial reptile. Behav Ecol Sociobiol 77, 29 (2023). https://doi.org/10.1007/s00265-023-03305-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03305-x