Abstract
Background
Adoptive tumor-infiltrating lymphocytes (TIL) therapy and interleukin-2 (IL-2) have been investigated in melanoma.
Aim
To confirm previously observed preventive effects of TIL + IL2 in a subgroup of patients with relapsing metastatic stage III melanoma.
Methodology
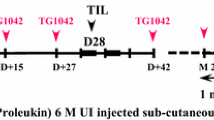
Open-label, randomized two-group, multicenter five-year trial in adult stage III melanoma patients with only one invaded lymph node after complete resection. Patients received TIL + IL2 or abstention. TIL + IL2 was administered within 8 weeks after lymph node resection and 4 weeks after. Disease-free survival was assessed every 2 months up to month 18, every 3 months up to month 36 and every 4 months up to 5 years. A once-a-year follow-up was scheduled beyond the five-year follow-up. Safety was assessed throughout the trial.
Results
Overall, 49 patients accounted for the modified intent-to-treat and 47 for the PP. Slightly more male than female patients participated; mean age was 57.7 ± 11.4 years in the TIL + IL2 group and 53.5 ± 13.0 years in the abstention group. After 5 years of follow-up, 11/26 patients in the TIL + IL2 group and 13/23 in the abstention group had relapsed. There was no statistical difference between the groups (HR: 0.63 CI 95% [0.28–1.41], p = 0.258), nine patients in the TIL + IL2 and 11 in the abstention group died with no significant difference between the two groups (HR: 0.65 CI95% [0.27 − 1.59], p = 0.34). Safety was good.
Conclusion
We did not confirm results of a previous trial. However, ulceration of the primary melanoma may be considered predictive of the efficacy of TIL in melanoma in adjuvant setting, in a manner similar to interferon α.




Similar content being viewed by others
References
Luke JJ, Flaherty KT, Ribas A, Long GV (2017) Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol 14(8):463–482
Svedman FC, Pillas D, Taylor A, Kaur M, Linder R, Hansson J (2016) Stage-specific survival and recurrence in patients with cutaneous malignant melanoma in Europe—a systematic review of the literature. Clin Epidemiol 8:109–122
Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL et al (2017) Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377(14):1345–1356
Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE et al (1985) Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 313(23):1485–1492
Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS et al (1994) Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst 86(15):1159–1166
Dudley ME, Wunderlich JR, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL et al (2002) A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother 25(3):243–251
Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ et al (2002) Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298(5594):850–854
Dudley ME, Gross CA, Langhan MM, Garcia MR, Sherry RM, Yang JC et al (2010) CD8+ enriched "young" tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res 16(24):6122–6131
Wu R, Forget MA, Chacon J, Bernatchez C, Haymaker C, Chen JQ et al (2012) Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer J 18(2):160–175
Andersen R, Donia M, Ellebaek E, Borch TH, Kongsted P, Iversen TZ et al (2016) long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated IL2 regimen. Clin Cancer Res 22(15):3734–3745
Dreno B, Nguyen JM, Khammari A, Pandolfino MC, Tessier MH, Bercegeay S et al (2002) Randomized trial of adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol Immunother 51(10):539–546
Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R et al (2014) Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother 63(7):721–735
Khammari A, Nguyen JM, Pandolfino MC, Quereux G, Brocard A, Bercegeay S et al (2007) Long-term follow-up of patients treated by adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol Immunother 56(11):1853–1860
Khammari A, Knol AC, Nguyen JM, Bossard C, Denis MG, Pandolfino MC et al (2014) Adoptive TIL transfer in the adjuvant setting for melanoma: long-term patient survival. J Immunol Res 2014:186212
Lotze MT, Frana LW, Sharrow SO, Robb RJ, Rosenberg SA (1985) In vivo administration of purified human interleukin 2. I. Half-life and immunologic effects of the Jurkat cell line-derived interleukin 2. J Immunol 134(1):157–66
Lotze MT, Matory YL, Ettinghausen SE, Rayner AA, Sharrow SO, Seipp CA et al (1985) In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J Immunol 135(4):2865–75
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5(6):649–655
Karnofsky D, Burchenal J (1949) Evaluation of chemotherapeutic agents in cancer. MacLoad, editor. Columbia University Press, New York
Keung EZ, Gershenwald JE (2018) The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: implications for melanoma treatment and care. Expert Rev Anticancer Ther 18(8):775–784
Jotereau F, Pandolfino MC, Boudart D, Diez E, Dreno B, Douillard JY et al (1991) High-fold expansion of human cytotoxic T-lymphocytes specific for autologous melanoma cells for use in immunotherapy. J Immunother 10(6):405–411
Pandolfino MC, Labarriere N, Tessier MH, Cassidanius A, Bercegeay S, Lemarre P et al (2001) High-scale expansion of melanoma-reactive TIL by a polyclonal stimulus: predictability and relation with disease advancement. Cancer Immunol Immunother 50(3):134–140
Tessier MH, Pandolfino MC, Jotereau F, Boudart D, Litoux P, Dreno B (1996) Home therapy with autologous tumor-infiltrating lymphocytes and subcutaneous interleukin-2 in metastatic melanoma. Eur J Cancer Engl 32a:735–736
Gervois N, Heuze F, Diez E, Jotereau F (1990) Selective expansion of a specific anti-tumor CD8+ cytotoxic T lymphocyte clone in the bulk culture of tumor-infiltrating lymphocytes from a melanoma patient: cytotoxic activity and T cell receptor gene rearrangements. Eur J Immunol 20(4):825–831
Pandolfino MC, Saiagh S, Knol AC, Dreno B (2010) Comparison of three culture media for the establishment of melanoma cell lines. Cytotechnology 62(5):403–412
Chebassier N, El Houssein O, Viegas I, Dreno B (2004) In vitro induction of matrix metalloproteinase-2 and matrix metalloproteinase-9 expression in keratinocytes by boron and manganese. Exp Dermatol 13(8):484–490
Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E et al (2002) Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U.S.A 99(25):16168–16173
Labarriere N, Pandolfino MC, Gervois N, Khammari A, Tessier MH, Dreno B et al (2002) Therapeutic efficacy of melanoma-reactive TIL injected in stage III melanoma patients. Cancer Immunol Immunother 51(10):532–538
Benlalam H, Vignard V, Khammari A, Bonnin A, Godet Y, Pandolfino MC et al (2007) Infusion of Melan-A/Mart-1 specific tumor-infiltrating lymphocytes enhanced relapse-free survival of melanoma patients. Cancer Immunol Immunother 56(4):515–526
Vignard V, Lemercier B, Lim A, Pandolfino MC, Guilloux Y, Khammari A et al (2005) Adoptive transfer of tumor-reactive Melan-A-specific CTL clones in melanoma patients is followed by increased frequencies of additional Melan-A-specific T cells. J Immunol 175(7):4797–4805
Zippel D, Friedman-Eldar O, Rayman S, Hazzan D, Nissan A, Schtrechman G et al (2019) Tissue harvesting for adoptive tumor infiltrating lymphocyte therapy in metastatic melanoma. Anticancer Res 39(9):4995–5001
Eggermont AMM, Robert C, Suciu S (2018) Adjuvant pembrolizumab in resected stage III melanoma. N Engl J Med U S 379:593–595
Jewell R, Elliott F, Laye J, Nsengimana J, Davies J, Walker C et al (2015) The clinicopathological and gene expression patterns associated with ulceration of primary melanoma. Pigment Cell Melanoma Res 28(1):94–104
Rohaan MW, van den Berg JH, Kvistborg P, Haanen JBAG (2018) Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: a viable treatment option. J Immunother Cancer 6(1):102
Zhang X, Zhao Y, Ye Y, Li S, Qi S, Yang Y et al (2015) Lesional infiltration of mast cells, Langerhans cells, T cells and local cytokine profiles in alopecia areata. Arch Dermatol Res 307(4):319–331
Gonzalez R, Torres-Lopez E (2014) Immunological basis of melanoma-associated vitiligo-like depigmentation. Actas Dermosifiliogr 105(2):122–127
Boniface K, Seneschal J, Picardo M, Taieb A (2018) Vitiligo: focus on clinical aspects, immunopathogenesis, and therapy. Clin Rev Allergy Immunol 54(1):52–67
Acknowledgements
The authors acknowledge the participation of the patients in this clinical trial and the writing and editing support of Karl Patrick Göritz, SMWS—Scientific and Medical Writing Services, France.
Funding
This study was performed within the LabEX IGO program, supported by the National Research Agency via the investment of the future program ANR-11-LABX-0016-01. Chiron France kindly provided interleukin-2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The trial complied with all French (AgenceNationale de Sécurité du Médicament et des Produits de Santé, ANSM,” Loi n° 2012–300, 5 Mars2012 relative aux recherchesimpliquant la personnehumaine” and all its amendments) and European requirements for the conduct of clinical trials (EuroepanMedicinec Agency, EMA, CPMP/ICH/377/95, EMEA) and the principles of Good Clinical Practices, the 1964 Declaration of Helsinki, received approval for all study sites from the ethics committee of Nantes, France in December 2003 (CPP Ouest IV Nantes n° 553/2005) and is registered at ClinicalTrials.gov under the Identifier: NCT00200577. All patients provided written informed consent prior to inclusion.
Conflict of interest
The authors have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper reports results from an open-label, randomized two-group, multicenter five-year trial in adult stage III melanoma patients with only one invaded lymph node after complete resection.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khammari, A., Nguyen, JM., Leccia, MT. et al. Tumor infiltrating lymphocytes as adjuvant treatment in stage III melanoma patients with only one invaded lymph node after complete resection: results from a multicentre, randomized clinical phase III trial. Cancer Immunol Immunother 69, 1663–1672 (2020). https://doi.org/10.1007/s00262-020-02572-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02572-1