Abstract
Background
The lethal effects of multiple antigen-specific cellular therapy (MASCT) may be enhanced by blocking PD-1 in vitro and vascular endothelial growth factor receptor 2 inhibitor (apatinib). We analyzed the pooled data from our phase I/II trials to determine the toxicity and efficacy of PD-1 blockade (SHR-1210)-activated MASCT (aMASCT) alone or in combination with apatinib in advanced solid tumors.
Methods
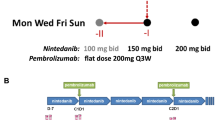
Patients with advanced solid tumors received aMASCT alone (n = 32) or aMASCT plus apatinib (500 mg q.d., n = 38) after standard treatment. The safety profile was the primary end point. The secondary end points were antitumor response, progression-free survival (PFS), and overall survival (OS). The circulating T cells were quantified before and after aMASCT infusion.
Results
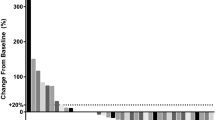
Treatment-related adverse events (AEs) occurred in 18/32 (56.3%) and 25/38 (65.8%) patients in the aMASCT and aMASCT plus apatinib groups, respectively. No serious AEs were reported, and apatinib did not increase immunotherapy-related toxicity. The objective response rate (34.2% and 18.8%) and PFS (median 6.0 and 4.5 months, P = 0.002) were improved in the aMASCT plus apatinib group compared with the aMASCT group; however, the OS was not improved (median 10.0 and 8.2 months, P = 0.098). Multivariate analyses indicated that two or more cycles of aMASCT treatment was an independent and favorable prognostic factor of PFS and OS. The circulating T cells increased and Tregs decreased in both groups after one cycle of aMASCT treatment.
Conclusions
Treatment with aMASCT plus apatinib was safe and effective for the management of advanced solid tumors.



Similar content being viewed by others
Abbreviations
- ACT:
-
Adoptive cellular therapy
- AEs:
-
Adverse events
- CI:
-
Confidence intervals
- CR:
-
Complete response
- CTL:
-
Cytotoxic T lymphocytes
- DCs:
-
Dendritic cells
- DCR:
-
Disease control rate
- PD:
-
Disease progression
- ELISPOT:
-
Enzyme-linked immunospot assay
- HFSR:
-
Hand–foot syndrome
- HR:
-
Hazard ratios
- MASCT:
-
Multiple antigen-specific cellular therapy
- MDSC:
-
Myeloid-derived suppression cells
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PR:
-
Partial response
- aMASCT:
-
PD-1 blockade (SHR-1210)-activated MASCT
- PBMCs:
-
Peripheral blood mononuclear cells
- PD-1:
-
Programmed cell death-1
- PD-L1:
-
Programmed cell death ligand-1
- PFS:
-
Progression-free survival
- Tregs:
-
Regulatory T cells
- RECIST:
-
Response Evaluation Criteria in Solid Tumors version 1.1
- SD:
-
Stable disease
- VEGF:
-
Vascular endothelial growth factor
- VEGFR2:
-
Vascular endothelial growth factor receptor 2
References
Liang L, Wen Y, Hui K, Jiang X (2019) Safety and efficacy of PD-1 blockade-activated multiple antigen specific cellular therapy alone or in combination with apatinib in patients with advanced solid tumors: A pooled analysis of two prospective trials. ASCO Annual Meeting 2019. J Clin Oncol 37 (suppl; abstract e14014)
Khalil DN, Smith EL, Brentjens RJ, Wolchok JD (2016) The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol 13(5):273–290. https://doi.org/10.1038/nrclinonc.2016.25
Kim JM, Chen DS (2016) Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Ann Oncol 27(8):1492–1504. https://doi.org/10.1093/annonc/mdw217
Teng MWL, Ngiow SF, Ribas A, Smyth MJ (2015) Classifying cancers based on T-cell infiltration and PD-L1. Can Res 75(11):2139–2145. https://doi.org/10.1158/0008-5472.can-15-0255
Yu H, Jove R (2004) The STATs of cancer–new molecular targets come of age. Nat Rev Cancer 4(2):97–105. https://doi.org/10.1038/nrc1275
Han Y, Wu Y, Yang C, Huang J, Guo Y, Liu L, Chen P, Wu D, Liu J, Li J, Zhou X, Hou J (2017) Dynamic and specific immune responses against multiple tumor antigens were elicited in patients with hepatocellular carcinoma after cell-based immunotherapy. J Transl Med 15(1):64. https://doi.org/10.1186/s12967-017-1165-0
He Y, Guo Y, Chen J, Hu X, Li X, Kong Y, Zhang X, Zhou X, Liu L, Hou J (2018) Multiple antigen stimulating cellular therapy (MASCT) for hepatocellular carcinoma after curative treatment: a retrospective study. J Cancer 9(8):1385–1393. https://doi.org/10.7150/jca.23725
Zhou Q, Munger ME, Highfill SL, Tolar J, Weigel BJ, Riddle M, Sharpe AH, Vallera DA, Azuma M, Levine BL, June CH, Murphy WJ, Munn DH, Blazar BR (2010) Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood 116(14):2484–2493. https://doi.org/10.1182/blood-2010-03-275446
Poh SL, Linn YC (2016) Immune checkpoint inhibitors enhance cytotoxicity of cytokine-induced killer cells against human myeloid leukaemic blasts. Cancer Immunol Immunother 65(5):525–536. https://doi.org/10.1007/s00262-016-1815-8
Chen CL, Pan QZ, Weng DS, Xie CM, Zhao JJ, Chen MS, Peng RQ, Li DD, Wang Y, Tang Y, Wang QJ, Zhang ZL, Zhang XF, Jiang LJ, Zhou ZQ, Zhu Q, He J, Liu Y, Zhou FJ, Xia JC (2018) Safety and activity of PD-1 blockade-activated DC-CIK cells in patients with advanced solid tumors. Oncoimmunology 7(4):e1417721. https://doi.org/10.1080/2162402X.2017.1417721
Hicklin DJ, Ellis LM (2005) Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23(5):1011–1027. https://doi.org/10.1200/jco.2005.06.081
Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, Wang Z, Wang Q, Ouyang X, Yang Y, Ba Y, Liang J, Lin X, Luo D, Zheng R, Wang X, Sun G, Wang L, Zheng L, Guo H, Wu J, Xu N, Yang J, Zhang H, Cheng Y, Wang N, Chen L, Fan Z, Sun P, Yu H (2016) Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 34(13):1448–1454. https://doi.org/10.1200/JCO.2015.63.5995
Tian S, Quan H, Xie C, Guo H, Lu F, Xu Y, Li J, Lou L (2011) YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 102(7):1374–1380. https://doi.org/10.1111/j.1349-7006.2011.01939.x
Scott LJ (2018) Apatinib: a review in advanced gastric cancer and other advanced cancers. Drugs 78(7):747–758. https://doi.org/10.1007/s40265-018-0903-9
Liang L, Wang L, Zhu P, Xia Y, Qiao Y, Wu J, Zhuang W, Fei J, Wen Y, Jiang X (2018) A pilot study of apatinib as third-line treatment in patients with heavily treated metastatic colorectal cancer. Clin Colorectal Cancer. https://doi.org/10.1016/j.clcc.2018.02.011
Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA (2010) Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Can Res 70(15):6171–6180. https://doi.org/10.1158/0008-5472.can-10-0153
Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, Gabrilovich DI (1998) Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol 160(3):1224–1232
Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK (2011) Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 91(3):1071–1121. https://doi.org/10.1152/physrev.00038.2010
Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, Lal P, Feldman MD, Benencia F, Coukos G (2014) Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med 20(6):607–615. https://doi.org/10.1038/nm.3541
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9(3):162–174. https://doi.org/10.1038/nri2506
Hu C, Jiang X (2017) The effect of anti-angiogenic drugs on regulatory T cells in the tumor microenvironment. Biomed Pharmacother 88:134–137. https://doi.org/10.1016/j.biopha.2017.01.051
Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378(24):2288–2301. https://doi.org/10.1056/NEJMoa1716948
Mo H, Huang J, Xu J, Chen X, Wu D, Qu D, Wang X, Lan B, Wang X, Xu J, Zhang H, Chi Y, Yang Q, Xu B (2018) Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study. Br J Cancer. https://doi.org/10.1038/s41416-018-0100-3
Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine BL, June CH, Schuster SJ (2017) PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood 129(8):1039–1041. https://doi.org/10.1182/blood-2016-09-738245
Zhou Y, Tang F, Min L, Zhang W, Shi R, Luo Y, Duan H, Tu C (2017) A preliminary study of apatinib in Chinese patients with osteosarcoma. J Clin Oncol 35(15_suppl):e22500–e22500. https://doi.org/10.1200/jco.2017.35.15_suppl.e22500
(2019) Institute NC surveillance, epidemiology, and end results program (SEER) cancer stat facts. https://seer.cancer.gov/statfacts/. Accessed 30 Mar 2019
Jiang N, Qiao G, Wang X, Morse MA, Gwin WR, Zhou L, Song Y, Zhao Y, Chen F, Zhou X, Huang L, Hobeika A, Yi X, Xia X, Guan Y, Song J, Ren J, Lyerly HK (2017) Dendritic cell/cytokine-induced killer cell immunotherapy combined with S-1 in patients with advanced pancreatic cancer: a prospective study. Clin Cancer Res 23(17):5066–5073. https://doi.org/10.1158/1078-0432.CCR-17-0492
Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, Korenstein D (2018) Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ 360:k793. https://doi.org/10.1136/bmj.k793
Deng W, Qin S, Li J, Wen L, Wang J, Zhang G, Zhong H, Yang J, Ba Y, Bai Y, Lin X, Wang M, Wang L, Liu L, He Y, Tao M, Xie C, Ye F, Wu X-y, Jia T (2018) Initial dose of apatinib in Chinese patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of stomach or gastroesophageal junction in third- or later-line setting: 500 mg or 850 mg? J Clin Oncol 36(4_suppl):35. https://doi.org/10.1200/jco.2018.36.4_suppl.35
Acknowledgements
We thank the patients enrolled in our clinical trials.
Funding
This work was supported by the Jiangsu Province Key Research and Development Plan (Social Development) Project (BE2017684), Scientific Research Project of Jiangsu Health and Family Planning Commission (H2017039), National Natural Science Foundation of China grants 81472792 and 81702813, Postgraduate Research & Practice Innovation Program of Jiangsu Province grants SJCX18_0707 and SJCX19_0766, the Technology Office Foundation of Lianyungang City (No. SH1613), and Beijing Medical and Health Foundation grants YWJKJJHKYJJ-13185016 and 13185014.
Author information
Authors and Affiliations
Contributions
XJ, LL, YW, and KH participated in the conception and design of this study. LW, YX, YQ, TC, JF, and XJ contributed to the recruitment of patients and acquisition and analysis and review of the data. LL, YW, RH, and XG conducted the in vitro experiments, analyzed the data, and wrote the manuscript. XJ and KH supervised the drafting of the manuscript. All authors critically reviewed each draft and approved the version to be published.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
The two single-center, open-label, phase I/II studies were conducted using a protocol approved by the medical ethics committee of the Affiliated Lianyungang Hospital of Xuzhou Medical University (2016016 and 2016018). All methods and procedures associated with this study were conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines, the Declaration of Helsinki, and the Chinese law. These trials were registered with the Clinical Trials Registry (NCT02844881, NCT02858232).
Informed consent
Informed consent for protocol therapy, the analysis of blood samples for research purposes, and the use of generated data for publication was obtained from all patients enrolled in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Parts of this paper were published as abstract no. e14014 at the American Society of Clinical Oncology (ASCO) Annual Meeting, 31 May–4 June 2019, Chicago, IL, USA [1].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liang, L., Wen, Y., Hu, R. et al. Safety and efficacy of PD-1 blockade-activated multiple antigen-specific cellular therapy alone or in combination with apatinib in patients with advanced solid tumors: a pooled analysis of two prospective trials. Cancer Immunol Immunother 68, 1467–1477 (2019). https://doi.org/10.1007/s00262-019-02375-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-019-02375-z