Abstract
Purpose
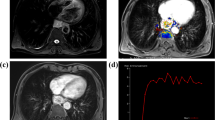
To compare conventional 3D volumetric-interpolated breath-hold examination (C-VIBE) sequence image quality to that of golden-angle radial stack-of stars acquisition scheme (R-VIBE) in rectal cancer patients.
Methods
Seventy-eight patients had undergone pre-contrast C-VIBE, followed by DCE-MRI with R-VIBE and post-contrast C-VIBE in the visualization of rectal cancer. The first phase and the last phase of R-VIBE sequence were compared with pre-contrast and post-contrast C-VIBE sequences, respectively. Signal-to-noise ratios (SNRs) and contrast-to-noise ratios (CNRs) of rectal neoplasms, gluteus maximus, and subcutaneous fat were compared between the two different sequences. A further qualitative score system (graded 1–5) was used to evaluate the overall image. Quantitative and qualitative parameters from the two sequences were compared.
Results
In all patients, R-VIBE achieved the same SNR and CNR ratings in pre- and post-contrast (all P > 0.05), with the exception of a higher SNR of fat in pre-contrast images (P = 0.037). In addition, there were no significant differences in scores of overall image quality, lesion conspicuity, and rectal wall boundary (all P > 0.05). There was an improved score in artifacts of post-contrast R-VIBE sequence (P = 0.005).
Conclusion
R-VIBE sequence can provide comparable image quality and less motion artifacts to that of C-VIBE sequence and is feasible for imaging of rectal cancer.



Similar content being viewed by others
Abbreviations
- MRI:
-
Magnetic resonance imaging
- DCE:
-
Dynamic contrast-enhanced
- VIBE:
-
Volumetric-interpolated breath-hold examination
- SNR:
-
Signal-to-noise ratio
- CNR:
-
Contrast-to-noise ratio
- CAIPIRINHA:
-
Controlled aliasing in parallel imaging results in higher acceleration
- GRAPPA:
-
Generalized autocalibrating partially parallel acquisitions
- TWIST:
-
Time-resolved imaging with stochastic trajectories
- ROI:
-
Region of interest
- SD:
-
Standard deviations
- RESOLVE:
-
Readout segmentation of long variable echo-trains
- CRT:
-
Chemoradiation therapy
- NCRT:
-
Neoadjuvant chemoradiation therapy
References
McKenzie CA, Lim D, Ransil BJ et al (2004) Shortening MR image acquisition time for volumetric interpolated breath-hold examination with a recently developed parallel imaging reconstruction technique: clinical feasibility. Radiology 230:589-94. https://doi.org/10.1148/radiol.2302021230
Runge VM, Richter JK, Heverhagen JT (2017) Speed in Clinical Magnetic Resonance. Invest Radiol 52:1-17. https://doi.org/10.1097/RLI.0000000000000330
Chandarana H, Block KT, Winfeld MJ et al (2014) Free-breathing contrast-enhanced T1-weighted gradient-echo imaging with radial k-space sampling for paediatric abdominopelvic MRI, Eur Radiol 24:320-326. https://doi.org/10.1007/s00330-013-3026-4
Heacock L, Gao Y, Heller SL et al (2017) Comparison of Conventional DCE-MRI and a Novel Golden-Angle Radial Multicoil Compressed Sensing Method for the Evaluation of Breast Lesion Conspicuity. J Magn Reson Imaging 45:1746-1752. https://doi.org/10.1002/jmri.25530
Rosenkrantz AB, Khasgiwala A, Doshi AM, Ream JM, Taneja SS, Lepor H (2017) Detection of prostate cancer local recurrence following radical prostatectomy: assessment using a continuously acquired radial golden-angle compressed sensing acquisition. Abdom Radiol 42:290-297. https://doi.org/10.1007/s00261-016-0881-x
Espagnet MCR, Bangiyev L, Haber M et al (2015) High-Resolution DCE-MRI of the Pituitary Gland Using Radial k-Space Acquisition with Compressed Sensing Reconstruction.Am J Neuroradiol 36:1444-1449. https://doi.org/10.3174/ajnr.A4324
Li HH, Zhu H, Yue L et al (2018) Feasibility of free-breathing dynamic contrast-enhanced MRI of gastric cancer using a golden-angle radial stack-of-stars VIBE sequence: comparison with the conventional contrast-enhanced breath-hold 3D VIBE sequence. Eur Radiol 28:1891-1899.https://doi.org/10.1007/s00330-017-5193-1
Chen W, Zheng R, Baade PD et al (2016) Cancer Statistics in China, 2015, Ca-Cancer. J Clin 66:115-132. https://doi.org/10.3322/caac.21338
Turkbey B, Thomasson D, Pang Y, Bernardo M, Choyke PL (2010) The role of dynamic contrast-enhanced MRI in cancer diagnosis and treatment. Diagn Interv Radiol 16:186-192. https://doi.org/10.4261/1305-3825.DIR.2537-08.1
Sun Y, Hu P, Wang J et al (2018) Radiomic features of pretreatment MRI could identify T stage in patients with rectal cancer: Preliminary findings. J Magn Reson Imaging. 48:615-621. https://doi.org/10.1002/jmri.25969
Gollub MJ, Cao K, Gultekin DH et al (2013) Prognostic Aspects of DCE-MRI in Recurrent Rectal Cancer. Eur Radiol 12: 3336-3344. https://doi.org/10.1007/s00330-013-2984-x
Ciolina M,Caruso D, De Santis et al (2019) Dynamic contrast-enhanced magnetic resonance imaging in locally advanced rectal cancer: role of perfusion parameters in the assessment of response to treatment. Radial Med 5:331-338. https://doi.org/10.1007/s11547-018-0978-0
Yang X, Chen Y, Wen Z et al (2019) Role of Quantitative Dynamic Contrast-Enhanced MRI in Evaluating Regional Lymph Nodes With a Short-Axis Diameter of Less Than 5 mm in Rectal Cancer. AJR Am J Roentgenol. 212:77-83. https://doi.org/10.2214/AJR.18.19866
Grøvik E, Redalen KR, Storås TH et al (2017) Dynamic multi-echo DCE- and DSC-MRI in rectal cancer: Low primary tumor Ktrans and ∆R2*peak are significantly associated with lymph node metastasis. J Magn Reson Imaging 46:194-206. https://doi.org/10.1002/jmri.25566
Gollub MJ, Gultekin DH, Akin O et al (2012) Dynamic contrast enhanced-MRI for the detection of pathological complete response to neoadjuvant chemotherapy for locally advanced rectal cancer. Eur Radiol 22:821-831. https://doi.org/10.1007/s00330-011-2321-1
Tong T, Sun Y, Gollub MJ et al (2015) Dynamic contrast-enhanced MRI: Use in predicting pathological complete response to neoadjuvant chemoradiation in locally advanced rectal cancer. J Magn Reson Imaging 42:673-680. https://doi.org/10.1002/jmri.24835
Intven M, Reerink O, Philippens MEP (2015) Dynamic contrast enhanced MR imaging for rectal cancer response assessment after neo-adjuvant chemoradiation. J Magn Reson Imaging 41:1646-1653. https://doi.org/10.1002/jmri.24718
Kim SH, Lee JM, Gupta SN, Han JK, Choi BI (2014) Dynamic Contrast-Enhanced MRI to Evaluate the Therapeutic Response to Neoadjuvant Chemoradiation Therapy in Locally Advanced Rectal Cancer. J Magn Reson Imaging 40:730-737. https://doi.org/10.1002jmri.24387
Heisen M, Fan X, Buurman J, van Riel NA, Karczmar GS, ter Haar Romeny BM (2010) The influence of temporal resolution in determining pharmacokinetic parameters from DCE-MRI data. Magn Reson Med 63:811-816. https://doi.org/10.1002/mm.22171
Grovik E, Redalen KR, Storas TH et al (2017) Dynamic multi-echo DCE- and DSC-MRI in rectal cancer: Low primary tumor K-trans and R2*peak are significantly associated with lymph node metastasis. J Magn Reson Imaging 46:194-206. https://doi.org/10.1002/jmri.25566
Nougaret S, Reinhold C, Mikhael HW, Rouanet P, Bibeau F, Brown G (2013) The Use of MR Imaging in Treatment Planning for Patients with Rectal Carcinoma: Have You Checked the “DISTANCE”?. RADIOLOGY 2:330-344. https://doi.org/10.1148/radiol.13121361
Tian Y, Erb KC, Adluru G et al (2017) Technical Note: Evaluation of pre-reconstruction interpolation methods for iterative reconstruction of radial k-space data. Med Phys 44:4025-4034. https://doi.org/10.1002/mp.12357
Shin HJ, Kim MJ, Lee MJ, Kim HG (2016) Comparison of image quality between conventional VIBE and radial VIBE in free-breathing paediatric abdominal MRI. Clin Radiol 71:1044-1049. https://doi.org/10.1016/j.crad.2016.03.018
Yedururi S, Kang HC, Wei W et al (2016) Free-breathing radial volumetric interpolated breath-hold examination vs breath-hold cartesian volumetric interpolated breath-hold examination magnetic resonance imaging of the liver at 1.5T. World J Radiol 8:707-715. https://doi.org/10.4329/wjr.v8.i7.707
Bamrungchart S, Tantaway EM, Midia EC et al (2013) Free breathing three-dimensional gradient echo-sequence with radial data sampling (radial 3D-GRE) examination of the pancreas: Comparison with standard 3D-GRE volumetric interpolated breathhold examination (VIBE). J Magn Reson Imaging 38:1572-1577. https://doi.org/10.1002/jmri.24064
Zhang XM, Yu D, Zhang HL et al (2008) 3D dynamic contrast-enhanced MRI of rectal carcinoma at 3T: correlation with microvascular density and vascular endothelial growth factor markers of tumor angiogenesis. J Magn Reson Imaging 27:1309-1316. https://doi.org/10.1002/jmri.21378
Hotker AM, Tarlinton L, Mazaheri Y et al (2016) Multiparametric MRI in the assessment of response of rectal cancer to neoadjuvant chemoradiotherapy: A comparison of morphological, volumetric and functional MRI parameters. Eur Radiol 26:4303-4312. https://doi.org/10.1007/s00330-016-4283-9
Dijkhoff RAP, Maas M, Martens MH et al (2017) Correlation between quantitative and semiquantitative parameters in DCE-MRI with a blood pool agent in rectal cancer: can semiquantitative parameters be used as a surrogate for quantitative parameters? Abdom Radiol 42:1342-1349. https://doi.org/10.1007/s00261-016-1024-0
Lollert A, Junginger T, Schimanski CC et al (2014) Rectal cancer: dynamic contrast-enhanced MRI correlates with lymph node status and epidermal growth factor receptor expression. J Magn Reson Imaging 39:1436-1442. https://doi.org/10.1002/jmri.24301
Kim YE, Lim JS, Choi J et al (2013) Perfusion parameters of dynamic contrast-enhanced magnetic resonance imaging in patients with rectal cancer: correlation with microvascular density and vascular endothelial growth factor expression. Korean J Radiol 14: 878-885. https://doi.org/10.3348/kjr.2013.14.6.878
Yeo D-M, Oh SN, Jung C-K et al (2015) Correlation of Dynamic Contrast-Enhanced MRI Perfusion Parameters With Angiogenesis and Biologic Aggressiveness of Rectal Cancer: Preliminary Results. J Magn Reson Imaging 41:474-480. https://doi.org/10.1002/jmri.24541
Hong H-S, Kim SH, Park H-J et al (2013) Correlations of Dynamic Contrast-Enhanced Magnetic Resonance Imaging with Morphologic, Angiogenic, and Molecular Prognostic Factors in Rectal Cancer. Yonsei Med J 54:123-130. https://doi.org/10.3349/ymj.2013.54.1.123
Petrillo M, Fusco R, Catalano O et al (2015) MRI for Assessing Response to Neoadjuvant Therapy in Locally Advanced Rectal Cancer Using DCE-MR and DW-MR Data Sets: A Preliminary Report. Biomed Res Int. https://doi.org/10.1155/2015/514740
Oberholzer K, Menig M, Pohlmann A et al (2013) Rectal cancer: assessment of response to neoadjuvant chemoradiation by dynamic contrast-enhanced MRI. J Magn Reson Imaging 38: 119-126. https://doi.org/10.1002.jmri.23952
Funding
This work was supported by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University [Grant Number ZYGD18019]; and multimodal MR imaging and Radiomics of rectal cancer, Science and technology department of Sichuan province [Grant Number 2019YFS0431].
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., Xia, C., Peng, W. et al. Dynamic contrast-enhanced MR imaging of rectal cancer using a golden-angle radial stack-of-stars VIBE sequence: comparison with conventional contrast-enhanced 3D VIBE sequence. Abdom Radiol 45, 322–331 (2020). https://doi.org/10.1007/s00261-019-02225-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-02225-7