Abstract
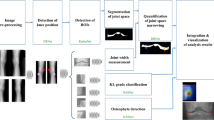
Deep learning (DL) is one of the most exciting new areas in medical imaging. This article will provide a review of current applications of DL in osteoarthritis (OA) imaging, including methods used for cartilage lesion detection, OA diagnosis, cartilage segmentation, and OA risk assessment. DL techniques have been shown to have similar diagnostic performance as human readers for detecting and grading cartilage lesions within the knee on MRI. A variety of DL methods have been developed for detecting and grading the severity of knee OA and various features of knee OA on X-rays using standardized classification systems with diagnostic performance similar to human readers. Multiple DL approaches have been described for fully automated segmentation of cartilage and other knee tissues and have achieved higher segmentation accuracy than currently used methods with substantial reductions in segmentation times. Various DL models analyzing baseline X-rays and MRI have been developed for OA risk assessment. These models have shown high diagnostic performance for predicting a wide variety of OA outcomes, including the incidence and progression of radiographic knee OA, the presence and progression of knee pain, and future total knee replacement. The preliminary results of DL applications in OA imaging have been encouraging. However, many DL techniques require further technical refinement to maximize diagnostic performance. Furthermore, the generalizability of DL approaches needs to be further investigated in prospective studies using large image datasets acquired at different institutions with different imaging hardware before they can be implemented in clinical practice and research studies.
Similar content being viewed by others
References
Erickson BJ, Korfiatis P, Akkus Z, Kline TL. Machine learning for medical imaging. Radiographics. 2017;37(2):505–15.
Binvignat M, Pedoia V, Butte AJ, Louati K, Klatzmann D, Berenbaum F, et al. Use of machine learning in osteoarthritis research: a systematic literature review. Rmd Open. 2022;8(1):e001998.
LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436–44.
Bedson J, Jordan K, Croft P. The prevalence and history of knee osteoarthritis in general practice: a case-control study. Fam Pract. 2005;22(1):103–8.
Chea P, Mandell JC. Current applications and future directions of deep learning in musculoskeletal radiology. Skeletal Radiol. 2020;49(2):183–97.
Goodfellow I , Bengio Y, Courville A. Deep learning. The MIT Press; 2016. p. 800. http://www.deeplearningbook.org.
Quatman CE, Hettrich CM, Schmitt LC, Spindler KP. The clinical utility and diagnostic performance of magnetic resonance imaging for identification of early and advanced knee osteoarthritis: a systematic review. Am J Sports Med. 2011;39(7):1557–68.
Menashe L, Hirko K, Losina E, Kloppenburg M, Zhang W, Li L, et al. The diagnostic performance of MRI in osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2012;20(1):13–21.
Liu F, Zhou Z, Samsonov A, Blankenbaker D, Larison W, Kanarek A, et al. Deep learning approach for evaluating knee MR images: achieving high diagnostic performance for cartilage lesion detection. Radiology. 2018;289(1):160–9.
Pedoia V, Norman B, Mehany SN, Bucknor MD, Link TM, Majumdar S. 3D convolutional neural networks for detection and severity staging of meniscus and PFJ cartilage morphological degenerative changes in osteoarthritis and anterior cruciate ligament subjects. J Magn Reson Imaging. 2019;49(2):400–10.
Astuto B, Flament I, Namiri NK, Shah R, Bharadwaj U, Link TM, et al. Automatic deep learning-assisted detection and grading of abnormalities in knee MRI studies. Radiol Artif Intell. 2021;3(3):e200165.
Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502.
Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl A):A1-56.
Gossec L, Jordan JM, Mazzuca SA, Lam MA, Suarez-Almazor ME, Renner JB, et al. Comparative evaluation of three semi-quantitative radiographic grading techniques for knee osteoarthritis in terms of validity and reproducibility in 1759 X-rays: report of the OARSI-OMERACT task force. Osteoarthritis Cartilage. 2008;16(7):742–8.
Sheehy L, Culham E, McLean L, Niu J, Lynch J, Segal NA, et al. Validity and sensitivity to change of three scales for the radiographic assessment of knee osteoarthritis using images from the Multicenter Osteoarthritis Study (MOST). Osteoarthritis Cartilage. 2015;23(9):1491–8.
Culvenor AG, Engen CN, Oiestad BE, Engebretsen L, Risberg MA. Defining the presence of radiographic knee osteoarthritis: a comparison between the Kellgren and Lawrence system and OARSI atlas criteria. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3532–9.
Antony J, McGuinness K, O'Connor NE, Moran K. Quantifying radiographic knee osteoarthritis severity using deep convolutional neural networks. In: 2016 23rd International Conference on Pattern Recognition (Icpr). 2016. https://doi.org/10.48550/arXiv.1609.02469.
Norman B, Pedoia V, Noworolski A, Link TM, Majumdar S. Applying densely connected convolutional neural networks for staging osteoarthritis severity from plain radiographs. J Digit Imaging. 2019;32(3):471–7.
Tiulpin A, Thevenot J, Rahtu E, Lehenkari P, Saarakkala S. Automatic knee osteoarthritis diagnosis from plain radiographs: a deep learning-based approach. Sci Rep. 2018;8(1):1727.
Thomas KA, Kidzinski L, Halilaj E, Fleming SL, Venkataraman GR, Oei EHG, et al. Automated classification of radiographic knee osteoarthritis severity using deep neural networks. Radiol Artif Intell. 2020;2(2):e190065.
Swiecicki A, Li N, O’Donnell J, Said N, Yang J, Mather RC, et al. Deep learning-based algorithm for assessment of knee osteoarthritis severity in radiographs matches performance of radiologists. Comput Biol Med. 2021;133:104334.
Kim DH, Lee KJ, Choi D, Lee JI, Choi HG, Lee YS. Can additional patient information improve the diagnostic performance of deep learning for the interpretation of knee osteoarthritis severity. J Clin Med. 2020;9(10):3341. https://doi.org/10.3390/jcm9103341.
Tiulpin A, Saarakkala S. Automatic grading of individual knee osteoarthritis features in plain radiographs using deep convolutional neural networks. Diagnostics (Basel). 2020;10(11):932. https://doi.org/10.3390/diagnostics10110932.
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–28.
Fripp J, Crozier S, Warfield SK, Ourselin S. Automatic segmentation and quantitative analysis of the articular cartilages from magnetic resonance images of the knee. IEEE Trans Med Imaging. 2010;29(1):55–64.
Tamez-Pena JG, Farber J, Gonzalez PC, Schreyer E, Schneider E, Totterman S. Unsupervised segmentation and quantification of anatomical knee features: data from the Osteoarthritis Initiative. IEEE Trans Biomed Eng. 2012;59(4):1177–86.
Prasoon A, Petersen K, Igel C, Lauze F, Dam E, Nielsen M. Deep feature learning for knee cartilage segmentation using a triplanar convolutional neural network. Med Image Comput Comput Assist Interv. 2013;16(Pt 2):246–53.
Liu F, Zhou Z, Jang H, Samsonov A, Zhao G, Kijowski R. Deep convolutional neural network and 3D deformable approach for tissue segmentation in musculoskeletal magnetic resonance imaging. Magn Reson Med. 2018;79(4):2379–91.
Zhou Z, Zhao G, Kijowski R, Liu F. Deep convolutional neural network for segmentation of knee joint anatomy. Magn Reson Med. 2018;80(6):2759–70.
Ambellan F, Tack A, Ehlke M, Zachow S. Automated segmentation of knee bone and cartilage combining statistical shape knowledge and convolutional neural networks: data from the Osteoarthritis Initiative. Med Image Anal. 2019;52:109–18.
Norman B, Pedoia V, Majumdar S. Use of 2D U-Net convolutional neural networks for automated cartilage and meniscus segmentation of knee MR imaging data to determine relaxometry and morphometry. Radiology. 2018;288(1):177–85.
Gaj S, Yang M, Nakamura K, Li X. Automated cartilage and meniscus segmentation of knee MRI with conditional generative adversarial networks. Magn Reson Med. 2020;84(1):437–49.
Yang M, Colak C, Chundru KK, Gaj S, Nanavati A, Jones MH, et al. Automated knee cartilage segmentation for heterogeneous clinical MRI using generative adversarial networks with transfer learning. Quant Imaging Med Surg. 2022;12(5):2620–33.
Kessler DA, MacKay JW, Crowe VA, Henson FMD, Graves MJ, Gilbert FJ, et al. The optimisation of deep neural networks for segmenting multiple knee joint tissues from MRIs. Comput Med Imaging Graph. 2020;86:101793.
Panfilov E, Tiulpin A, Nieminen MT, Saarakkala S, Casula V. Deep learning-based segmentation of knee MRI for fully automatic subregional morphological assessment of cartilage tissues: data from the osteoarthritis initiative. J Orthop Res. 2022;40(5):1113–24.
Wirth W, Eckstein F, Kemnitz J, Baumgartner CF, Konukoglu E, Fuerst D, et al. Accuracy and longitudinal reproducibility of quantitative femorotibial cartilage measures derived from automated U-Net-based segmentation of two different MRI contrasts: data from the osteoarthritis initiative healthy reference cohort. MAGMA. 2021;34(3):337–54.
Eckstein F, Chaudhari AS, Fuerst D, Gaisberger M, Kemnitz J, Baumgartner CF, et al. Detection of differences in longitudinal cartilage thickness loss using a deep-learning automated segmentation algorithm: data from the Foundation for the National Institutes of Health Biomarkers Study of the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2022;74(6):929–36.
Chen H, Zhao N, Tan T, Kang Y, Sun C, Xie G, et al. Knee bone and cartilage segmentation based on a 3D deep neural network using adversarial loss for prior shape constraint. Front Med (Lausanne). 2022;9:792900.
Desai AD, Caliva F, Iriondo C, Mortazi A, Jambawalikar S, Bagci U, et al. The International workshop on osteoarthritis imaging knee MRI segmentation challenge: a multi-institute evaluation and analysis framework on a standardized dataset. Radiol Artif Intell. 2021;3(3):e200078.
Christensen R, Astrup A, Bliddal H. Weight loss: the treatment of choice for knee osteoarthritis? A randomized trial. Osteoarthr Cartil / OARS, Osteoarthr Res Soc. 2005;13(1):20–7.
Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50(5):1501–10.
Christensen R, Bartels EM, Astrup A, Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2007;66(4):433–9.
Ettinger WH Jr, Burns R, Messier SP, Applegate W, Rejeski WJ, Morgan T, et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST). JAMA: J Am Med Assoc. 1997;277(1):25–31.
Roddy E, Zhang W, Doherty M. Aerobic walking or strengthening exercise for osteoarthritis of the knee? A systematic review. Ann Rheum Dis. 2005;64(4):544–8.
Roddy E, Zhang W, Doherty M, Arden NK, Barlow J, Birrell F, et al. Evidence-based recommendations for the role of exercise in the management of osteoarthritis of the hip or knee–the MOVE consensus. Rheumatology (Oxford). 2005;44(1):67–73.
Brosseau L, MacLeay L, Robinson V, Wells G, Tugwell P. Intensity of exercise for the treatment of osteoarthritis. Cochrane Database Syst Rev. 2003;(2):CD004259. https://doi.org/10.1002/14651858.CD004259.
Deyle GD, Henderson NE, Matekel RL, Ryder MG, Garber MB, Allison SC. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee. A randomized, controlled trial. Ann Intern Med. 2000;132(3):173–81.
Fransen M, McConnell S. Land-based exercise for osteoarthritis of the knee: a metaanalysis of randomized controlled trials. J Rheumatol. 2009;36(6):1109–17.
Fransen M, Crosbie J, Edmonds J. Physical therapy is effective for patients with osteoarthritis of the knee: a randomized controlled clinical trial. J Rheumatol. 2001;28(1):156–64.
Bang MD, Deyle GD. Comparison of supervised exercise with and without manual physical therapy for patients with shoulder impingement syndrome. J Orthop Sports Phys Ther. 2000;30(3):126–37.
Felson DT, Hodgson R. Identifying and treating preclinical and early osteoarthritis. Rheum Dis Clin North Am. 2014;40(4):699–710.
Eckstein F, Burstein D, Link TM. Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed. 2006;19(7):822–54.
Hunter DJ, Niu J, Zhang Y, Totterman S, Tamez J, Dabrowski C, et al. Change in cartilage morphometry: a sample of the progression cohort of the Osteoarthritis Initiative. Ann Rheum Dis. 2009;68(3):349–56.
Hunter DJ, Conaghan PG, Peterfy CG, Bloch D, Guermazi A, Woodworth T, et al. Responsiveness, effect size, and smallest detectable difference of magnetic resonance imaging in knee osteoarthritis. Osteoarthr Cartil / OARS, Osteoarthr Res Soc. 2006;14(Suppl A):A112-115.
Reichmann WM, Maillefert JF, Hunter DJ, Katz JN, Conaghan PG, Losina E. Responsiveness to change and reliability of measurement of radiographic joint space width in osteoarthritis of the knee: a systematic review. Osteoarthr Cartil. 2011;19(5):550–6.
Pelletier JP, Martel-Pelletier J, Raynauld JP. Most recent developments in strategies to reduce the progression of structural changes in osteoarthritis: today and tomorrow. Arthritis Res Ther. 2006;8(2):206.
Pelletier JP, Martel-Pelletier J. DMOAD developments: present and future. Bull NYU Hosp Jt Dis. 2007;65(3):242–8.
Qvist P, Bay-Jensen AC, Christiansen C, Dam EB, Pastoureau P, Karsdal MA. The disease modifying osteoarthritis drug (DMOAD): is it in the horizon? Pharmacol Res : Off J Ital Pharmacol Soc. 2008;58(1):1–7.
Hellio Le Graverand-Gastineau MP. OA clinical trials: current targets and trials for OA. Choosing molecular targets: what have we learned and where we are headed? Osteoarthr Cartil / OARS, Osteoarthr Res Soc. 2009;17(11):1393–401.
Hunter DJ, Hellio Le Graverand-Gastineau MP. How close are we to having structure-modifying drugs available? Rheum Dis Clin North Am. 2008;(3):789–802. https://doi.org/10.1016/j.rdc.2008.05.003.
Hunter DJ. Risk stratification for knee osteoarthritis progression: a narrative review. Osteoarthr Cartil. 2009;17(11):1402–7.
Hunter DJ, Nevitt M, Losina E, Kraus V. Biomarkers for osteoarthritis: current position and steps towards further validation. Best Pract Res Clin Rheumatol. 2014;28(1):61–71.
Felson D, Niu J, Sack B, Aliabadi P, McCullough C, Nevitt MC. Progression of osteoarthritis as a state of inertia. Ann Rheum Dis. 2013;72(6):924–9.
Leyland KM, Hart DJ, Javaid MK, Judge A, Kiran A, Soni A, et al. The natural history of radiographic knee osteoarthritis: a fourteen-year population-based cohort study. Arthritis Rheum. 2012;64(7):2243–51.
Oak SR, Ghodadra A, Winalski CS, Miniaci A, Jones MH. Radiographic joint space width is correlated with 4-year clinical outcomes in patients with knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthr Cartil. 2013;21(9):1185–90.
Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61(4):459–67.
Collins JE, Losina E, Nevitt MC, Roemer FW, Guermazi A, Lynch JA, et al. Semiquantitative imaging biomarkers of knee osteoarthritis progression: data from the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol. 2016;68(10):2422–31.
Roemer FW, Kwoh CK, Hannon MJ, Green SM, Jakicic JM, Boudreau R, et al. Risk factors for magnetic resonance imaging-detected patellofemoral and tibiofemoral cartilage loss during a six-month period: the joints on glucosamine study. Arthritis Rheum. 2012;64(6):1888–98.
Roemer FW, Guermazi A, Felson DT, Niu J, Nevitt MC, Crema MD, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70(10):1804–9.
Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis – results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthr Cartil / OARS, Osteoarthr Res Soc. 2005;13(5):361–7.
Atukorala I, Kwoh CK, Guermazi A, Roemer FW, Boudreau RM, Hannon MJ, et al. Synovitis in knee osteoarthritis: a precursor of disease? Ann Rheum Dis. 2016;75(2):390–5.
Crema MD, Guermazi A, Li L, Nogueira-Barbosa MH, Marra MD, Roemer FW, et al. The association of prevalent medial meniscal pathology with cartilage loss in the medial tibiofemoral compartment over a 2-year period. Osteoarthr Cartil / OARS, Osteoarthr Res Soc. 2010;18(3):336–43.
Chang A, Moisio K, Chmiel JS, Eckstein F, Guermazi A, Almagor O, et al. Subregional effects of meniscal tears on cartilage loss over 2 years in knee osteoarthritis. Ann Rheum Dis. 2011;70(1):74–9.
Wluka AE, Hanna F, Davies-Tuck M, Wang Y, Bell RJ, Davis SR, et al. Bone marrow lesions predict increase in knee cartilage defects and loss of cartilage volume in middle-aged women without knee pain over 2 years. Ann Rheum Dis. 2009;68(6):850–5.
Wluka AE, Wang Y, Davies-Tuck M, English DR, Giles GG, Cicuttini FM. Bone marrow lesions predict progression of cartilage defects and loss of cartilage volume in healthy middle-aged adults without knee pain over 2 yrs. Rheumatology. 2008;47(9):1392–6.
Dore D, Martens A, Quinn S, Ding C, Winzenberg T, Zhai G, et al. Bone marrow lesions predict site-specific cartilage defect development and volume loss: a prospective study in older adults. Arthritis Res Ther. 2010;12(6):R222.
Scher C, Craig J, Nelson F. Bone marrow edema in the knee in osteoarthrosis and association with total knee arthroplasty within a three-year follow-up. Skeletal Radiol. 2008;37(7):609–17.
Han W, Aitken D, Zhu Z, Halliday A, Wang X, Antony B, et al. Signal intensity alteration in the infrapatellar fat pad at baseline for the prediction of knee symptoms and structure in older adults: a cohort study. Ann Rheum Dis. 2016;75(10):1783–8.
Tiulpin A, Klein S, Bierma-Zeinstra SMA, Thevenot J, Rahtu E, Meurs JV, et al. Multimodal machine learning-based knee osteoarthritis progression prediction from plain radiographs and clinical data. Sci Rep. 2019;9(1):20038.
Guan B, Liu F, Haj-Mirzaian A, Demehri S, Samsonov A, Neogi T, et al. Deep learning risk assessment models for predicting progression of radiographic medial joint space loss over a 48-month follow-up period. Osteoarthritis Cartilage. 2020;28(4):428–37.
Schiratti JB, Dubois R, Herent P, Cahane D, Dachary J, Clozel T, et al. A deep learning method for predicting knee osteoarthritis radiographic progression from MRI. Arthritis Res Ther. 2021;23(1):262.
Leung K, Zhang B, Tan J, Shen Y, Geras KJ, Babb JS, et al. Prediction of total knee replacement and diagnosis of osteoarthritis by using deep learning on knee radiographs: data from the osteoarthritis initiative. Radiology. 2020;296(3):584–93.
Tolpadi AA, Lee JJ, Pedoia V, Majumdar S. Deep learning predicts total knee replacement from magnetic resonance images. Sci Rep. 2020;10(1):6371.
Wang T, Leung K, Cho K, Change G, Deniz C. Total knee replacement prediction using structural MRIs and 3D convolutional neural networks. In International Conference on Medical Imaging and Deep Learning- Extended Abstract Track; 2019;79.
Guan B, Liu F, Mizaian AH, Demehri S, Samsonov A, Guermazi A, et al. Deep learning approach to predict pain progression in knee osteoarthritis. Skeletal Radiol. 2022;51(2):363–73.
Chang GH, Felson DT, Qiu S, Guermazi A, Capellini TD, Kolachalama VB. Assessment of knee pain from MR imaging using a convolutional Siamese network. Eur Radiol. 2020;30(6):3538–48.
Lee J, Liu F, Majumdar S, Pedoia V. An ensemble clinical and MRI deep learning model predicts 8-year knee pain trajectory: data from the osteoarthritis initiative. Osteoarthr Imaging. 2021;1:1000003.
Pedoia V, Lee J, Norman B, Link TM, Majumdar S. Diagnosing osteoarthritis from T2 maps using deep learning: an analysis of the entire Osteoarthritis initiative baseline cohort. Osteoarthr Cartil. 2019;27(7):1002–10.
Allen-Zhu Z, Li Y. Towards understanding ensemble, knowledge distillation and self-distillation in deep learning. 2020. https://doi.org/10.48550/arXiv.2012.09816
Zhou T, Ruan S, Guo Y, Canu S. A multi-modality fusion network based on attention mechanism for brain tumor segmentation. In: 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI). 2020. pp. 377–380.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Deep learning techniques have similar diagnostic performance as human readers for detecting and grading cartilage lesions within the knee on MRI.
• Deep learning methods can detect and grade the severity of knee osteoarthritis and features of knee osteoarthritis on X-rays with similar diagnostic performance as human readers.
•Deep learning approaches for full-automated segmentation of cartilage and other knee tissue can achieve higher segmentation accuracy than currently used methods with substantial reductions in segmentation times.
•Deep learning models have high diagnostic performance for predicting osteoarthritis outcomes, including the incidence and progression of radiographic knee osteoarthritis, the presence and progression of knee pain, and future total knee replacement.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kijowski, R., Fritz, J. & Deniz, C.M. Deep learning applications in osteoarthritis imaging. Skeletal Radiol 52, 2225–2238 (2023). https://doi.org/10.1007/s00256-023-04296-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-023-04296-6