Abstract
The advancements of artificial intelligence (AI) for osteoarthritis (OA) applications have been rapid in recent years, particularly innovations of deep learning for image classification, lesion detection, cartilage segmentation, and prediction modeling of future knee OA development. This review article focuses on AI applications in OA research, first describing machine learning (ML) techniques and workflow, followed by how these algorithms are used for OA classification tasks through imaging and non-imaging-based ML models. Deep learning applications for OA research, including analysis of both radiographs for automatic detection of OA severity, and MR images for detection of cartilage/meniscus lesions and cartilage segmentation for automatic T2 quantification will be described. In addition, information on ML models that identify individuals at high risk of OA development will be provided. The future vision of machine learning applications in imaging of OA and cartilage hinges on implementation of AI for optimizing imaging protocols, quantitative assessment of cartilage, and automated analysis of disease burden yielding a faster and more efficient workflow for a radiologist with a higher level of reproducibility and precision. It may also provide risk assessment tools for individual patients, which is an integral part of precision medicine.


Similar content being viewed by others
References
Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–223.
Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112(3 Suppl 1):S13–9.
Weinstein AM, Rome BN, Reichmann WM, Collins JE, Burbine SA, Thornhill TS, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95(5):385–92.
Center ME-bP. Total knee replacement. Minneapolis: Agency for Healthcare and Research Quality; 2003.
Ghouri A, Conaghan PG. Update on novel pharmacological therapies for osteoarthritis. Ther Adv Musculoskelet Dis. 2019;11:1759720X19864492. https://doi.org/10.1177/1759720X19864492.
Dell’Isola A, Allan R, Smith SL, Marreiros SS, Steultjens M. Identification of clinical phenotypes in knee osteoarthritis: a systematic review of the literature. BMC Musculoskelet Disord. 2016;17(1):425.
Waarsing JH, Bierma-Zeinstra SM, Weinans H. Distinct subtypes of knee osteoarthritis: data from the Osteoarthritis Initiative. Rheumatology (Oxford). 2015;54(9):1650–8.
Deveza LA, Melo L, Yamato TP, Mills K, Ravi V, Hunter DJ. Knee osteoarthritis phenotypes and their relevance for outcomes: a systematic review. Osteoarthritis Cartilage. 2017;25(12):1926–41.
Roddy E, Doherty M. Changing life-styles and osteoarthritis: what is the evidence? Best Pract Res Clin Rheumatol. 2006;20(1):81–97.
Cooper C, Snow S, McAlindon TE, Kellingray S, Stuart B, Coggon D, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000;43(5):995–1000.
Musumeci G, Aiello FC, Szychlinska MA, Di Rosa M, Castrogiovanni P, Mobasheri A. Osteoarthritis in the XXIst century: risk factors and behaviours that influence disease onset and progression. Int J Mol Sci. 2015;16(3):6093–112.
Sharma L, Nevitt M, Hochberg M, Guermazi A, Roemer FW, Crema M, et al. Clinical significance of worsening versus stable preradiographic MRI lesions in a cohort study of persons at higher risk for knee osteoarthritis. Ann Rheum Dis. 2016;75(9):1630–6.
Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J, et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the Osteoarthritis Initiative. Arthritis Care Res. 2012;64(2):248–55.
Joseph GB, Baum T, Alizai H, Carballido-Gamio J, Nardo L, Virayavanich W, et al. Baseline mean and heterogeneity of MR cartilage T2 are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years–data from the Osteoarthritis Initiative. Osteoarthr Cartil. 2012;20(7):727–35.
Cabitza F, Locoro A, Banfi G. Machine learning in orthopedics: a literature review. Front Bioeng Biotechnol. 2018;6:75.
Kokkotis C, Moustakidis S, Papageorgiou E, Giakas G, Tsaopoulos DE. Machine learning in knee osteoarthritis: a review. Osteoarthritis and Cartilage Open. 2020;2(3):100069.
Hirschmann A, Cyriac J, Stieltjes B, Kober T, Richiardi J, Omoumi P. Artificial intelligence in musculoskeletal imaging: review of current literature, challenges, and trends. Semin Musculoskelet Radiol. 2019;23(3):304–11.
Joseph GB, McCulloch CE, Nevitt MC, Neumann J, Gersing AS, Kretzschmar M, et al. Tool for osteoarthritis risk prediction (TOARP) over 8 years using baseline clinical data, X-ray, and MRI: data from the osteoarthritis initiative. J Magn Reson Imaging. 2018;47(6):1517–26.
Norman B, Pedoia V, Noworolski A, Link TM, Majumdar S. Applying densely connected convolutional neural networks for staging osteoarthritis severity from plain radiographs. J Digit Imaging. 2019;32(3):471–7.
Furche T, Gottlob G, Libkin L, Orsi G, Paton NW. Data wrangling for big data: challenges and opportunities. InEDBT. 2016;16:473–8.
Rahmn AU. What is data cleaning? How to process data for analytics and machine learning modeling? : toward data science; 2019 Available from: https://towardsdatascience.com/what-is-data-cleaning-how-to-process-data-for-analytics-and-machine-learning-modeling-c2afcf4fbf45.
Jerez JM, Molina I, García-Laencina PJ, Alba E, Ribelles N, Martín M, et al. Missing data imputation using statistical and machine learning methods in a real breast cancer problem. Artif Intell Med. 2010;50(2):105–15.
Reddy GT, Reddy MPK, Lakshmanna K, Kaluri R, Rajput DS, Srivastava G, et al. Analysis of dimensionality reduction techniques on big data. IEEE Access. 2020;8:54776–88.
Jamshidi A, Pelletier JP, Martel-Pelletier J. Machine-learning-based patient-specific prediction models for knee osteoarthritis. Nat Rev Rheumatol. 2019;15(1):49–60.
Guo X, Yin Y, Dong C, Yang G, Zhou G. On the class imbalance problem. In: 2008 Fourth international conference on natural computation. IEEE; 2008. Vol. 4, pp. 192–201.
Chlap P, Min H, Vandenberg N, Dowling J, Holloway L, Haworth A. A review of medical image data augmentation techniques for deep learning applications. J Med Imaging Radiat Oncol. 2021;65(5):545–63.
Hugle M, Omoumi P, van Laar JM, Boedecker J, Hugle T. Applied machine learning and artificial intelligence in rheumatology. Rheumatol Adv Pract. 2020;4(1):rkaa005.
Webb GI, Keogh E, Miikkulainen R. Naïve Bayes. Encyclopedia of machine learning. 2010;15:713-4.
Singh A, Thakur N, Sharma A, editors. A review of supervised machine learning algorithms. 2016 3rd International Conference on Computing for Sustainable Global Development (INDIACom); 2016 16–18 March 2016.
Kherif F, Latypova A. Chapter 12 - Principal component analysis. In: Mechelli A, Vieira S, editors. Machine Learning. Academic Press; 2020. p. 209–25.
Kokkotis C, Moustakidis S, Papageorgiou E, Giakas G, Tsaopoulos DE. Machine learning in knee osteoarthritis: a review. Osteoarthr Cartil Open. 2020;2(3):100069.
Grootendorst M. Validating your machine learning model, going beyond k-fold cross-validation 2019 Available from: https://towardsdatascience.com/validating-your-machine-learning-model-25b4c8643fb7.
Narkhede S. Understanding AUC-ROC curve. Towards data science. 2018;26. https://towardsdatascience.com/understanding-auc-roc-curve-68b2303cc9c5.
Maroco J, Silva D, Rodrigues A, Guerreiro M, Santana I, de Mendonça A. Data mining methods in the prediction of dementia: a real-data comparison of the accuracy, sensitivity and specificity of linear discriminant analysis, logistic regression, neural networks, support vector machines, classification trees and random forests. BMC Res Notes. 2011;4(1):299.
Powers DM. Evaluation: from precision, recall and F-measure to ROC, informedness, markedness and correlation. arXiv preprint arXiv:201016061. 2020.
Ping Shung K. Accuracy, precision, recall or F1? https://towardsdatascience.com/accuracy-precision-recall-or-f1-331fb37c5cb9 [Internet]. 2018; 2021.
Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945;26(3):297–302.
Laber EB, Murphy SA, editors. Small sample inference for generalization error in classification using the CUD bound. Uncertainty in artificial intelligence: proceedings of the conference Conference on Uncertainty in Artificial Intelligence; 2008: NIH Public Access.
Stead WW. Clinical implications and challenges of artificial intelligence and deep learning. JAMA. 2018;320(11):1107–8.
Kerkhof HJ, Bierma-Zeinstra SM, Arden NK, Metrustry S, Castano-Betancourt M, Hart DJ, et al. Prediction model for knee osteoarthritis incidence, including clinical, genetic and biochemical risk factors. Ann Rheum Dis. 2014;73(12):2116–21.
Ashinsky BG, Bouhrara M, Coletta CE, Lehallier B, Urish KL, Lin PC, et al. Predicting early symptomatic osteoarthritis in the human knee using machine learning classification of magnetic resonance images from the osteoarthritis initiative. J Orthop Res. 2017;35(10):2243–50.
Štajduhar I, Mamula M, Miletić D, Ünal G. Semi-automated detection of anterior cruciate ligament injury from MRI. Comput Methods Programs Biomed. 2017;140:151–64.
Lazzarini N, Runhaar J, Bay-Jensen AC, Thudium CS, Bierma-Zeinstra SMA, Henrotin Y, et al. A machine learning approach for the identification of new biomarkers for knee osteoarthritis development in overweight and obese women. Osteoarthr Cartil. 2017;25(12):2014–21.
Halilaj E, Le Y, Hicks JL, Hastie TJ, Delp SL. Modeling and predicting osteoarthritis progression: data from the osteoarthritis initiative. Osteoarthr Cartil. 2018;26(12):1643–50.
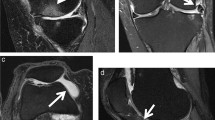
Liu F, Zhou Z, Samsonov A, Blankenbaker D, Larison W, Kanarek A, et al. Deep learning approach for evaluating knee MR images: achieving high diagnostic performance for cartilage lesion detection. Radiology. 2018;289(1):160–9.
Tiulpin A, Thevenot J, Rahtu E, Lehenkari P, Saarakkala S. Automatic knee osteoarthritis diagnosis from plain radiographs: a deep learning-based approach. Sci Rep. 2018;8(1):1727.
Bien N, Rajpurkar P, Ball RL, Irvin J, Park A, Jones E, et al. Deep-learning-assisted diagnosis for knee magnetic resonance imaging: development and retrospective validation of MRNet. PLoS Med. 2018;15(11):e1002699.
Du Y, Almajalid R, Shan J, Zhang M. A novel method to predict knee osteoarthritis progression on MRI using machine learning methods. IEEE Trans Nanobioscience. 2018;17(3):228–36.
Antony J. Automatic quantification of radiographic knee osteoarthritis severity and associated diagnostic features using deep convolutional neural networks (Doctoral dissertation, Dublin City University). 2018. http://doras.dcu.ie/22154/.
Pedoia V, Norman B, Mehany SN, Bucknor MD, Link TM, Majumdar S. 3D convolutional neural networks for detection and severity staging of meniscus and PFJ cartilage morphological degenerative changes in osteoarthritis and anterior cruciate ligament subjects. J Magn Reson Imaging. 2019;49(2):400–10.
Nelson AE, Fang F, Arbeeva L, Cleveland RJ, Schwartz TA, Callahan LF, et al. A machine learning approach to knee osteoarthritis phenotyping: data from the FNIH Biomarkers Consortium. Osteoarthr Cartil. 2019;27(7):994–1001.
Brahim A, Jennane R, Riad R, Janvier T, Khedher L, Toumi H, et al. A decision support tool for early detection of knee OsteoArthritis using X-ray imaging and machine learning: data from the OsteoArthritis Initiative. Comput Med Imaging Graph. 2019;73:11–8.
Roblot V, Giret Y, Bou Antoun M, Morillot C, Chassin X, Cotten A, et al. Artificial intelligence to diagnose meniscus tears on MRI. Diagn Interv Imaging. 2019;100(4):243–9.
Couteaux V, Si-Mohamed S, Nempont O, Lefevre T, Popoff A, Pizaine G, et al. Automatic knee meniscus tear detection and orientation classification with Mask-RCNN. Diagn Interv Imaging. 2019;100(4):235–42.
Abedin J, Antony J, McGuinness K, Moran K, O’Connor NE, Rebholz-Schuhmann D, et al. Predicting knee osteoarthritis severity: comparative modeling based on patient’s data and plain X-ray images. Sci Rep. 2019;9(1):5761.
Chang PD, Wong TT, Rasiej MJ. Deep learning for detection of complete anterior cruciate ligament tear. J Digit Imaging. 2019;32(6):980–6.
Pedoia V, Lee J, Norman B, Link TM, Majumdar S. Diagnosing osteoarthritis from T2 maps using deep learning: an analysis of the entire Osteoarthritis Initiative baseline cohort. Osteoarthritis Cartilage. 2019;27(7):1002–10.
Tiulpin A, Klein S, Bierma-Zeinstra SMA, Thevenot J, Rahtu E, Meurs JV, et al. Multimodal machine learning-based knee osteoarthritis progression prediction from plain radiographs and clinical data. Sci Rep. 2019;9(1):20038.
Jamshidi A, Leclercq M, Labbe A, Pelletier JP, Abram F, Droit A, et al. Identification of the most important features of knee osteoarthritis structural progressors using machine learning methods. Ther Adv Musculoskelet Dis. 2020;12:1759720X20933468.
Leung K, Zhang B, Tan J, Shen Y, Geras KJ, Babb JS, et al. Prediction of total knee replacement and diagnosis of osteoarthritis by using deep learning on knee radiographs: data from the Osteoarthritis Initiative. Radiology. 2020;296(3):584–93.
Widera P, Welsing PMJ, Ladel C, Loughlin J, Lafeber F, Petit Dop F, et al. Multi-classifier prediction of knee osteoarthritis progression from incomplete imbalanced longitudinal data. Sci Rep. 2020;10(1):8427.
Alexos A, Moustakidis S, Kokkotis C, Tsaopoulos D. Physical activity as a risk factor in the progression of osteoarthritis: a machine learning perspective. In: International Conference on Learning and Intelligent Optimization. Cham: Springer; 2020. pp. 16–26.
Thomas KA, Kidziński Ł, Halilaj E, Fleming SL, Venkataraman GR, Oei EH, et al. Automated classification of radiographic knee osteoarthritis severity using deep neural networks. Radiol Artif Intell. 2020;2(2):e190065.
Kwon SB, Ku Y, Han HU, Lee MC, Kim HC, Ro DH. A machine learning-based diagnostic model associated with knee osteoarthritis severity. Sci Rep. 2020;10(1):15743.
Guan B, Liu F, Haj-Mirzaian A, Demehri S, Samsonov A, Neogi T, et al. Deep learning risk assessment models for predicting progression of radiographic medial joint space loss over a 48-MONTH follow-up period. Osteoarthr Cartil. 2020;28(4):428–37.
Guan B, Liu F, Matthew P, Mirzaian AH, Demehri S, Neogi T, et al. Deep learning approach to predict pain progression in knee osteoarthritis. Osteoarthr Cartil. 2020;28:S316.
Tolpadi AA, Lee JJ, Pedoia V, Majumdar S. Deep learning predicts total knee replacement from magnetic resonance images. Sci Rep. 2020;10(1):6371.
Razmjoo A, Caliva F, Lee J, Liu F, Joseph GB, Link TM, et al. T2 analysis of the entire osteoarthritis initiative dataset. J Orthop Res. 2021;39(1):74–85.
Joseph GB, Mcculloch CE, Nevitt MC, Link TM, JH. S, editors. Machine learning for predicting knee osteoarthritis progression over 8 years using combined MR imaging features, demographics, and clinical factors: data from the Osteoarthritis Initiative World Congress on Osteoarthritis; 2021; Virtual due to Covid 19.
Gan H-S, Ramlee MH, Wahab AA, Lee Y-S, Shimizu A. From classical to deep learning: review on cartilage and bone segmentation techniques in knee osteoarthritis research. Artif Intell Rev. 2021;54(4):2445–94.
Kellgren J, Lawrence J. Radiologic assessment of osteoarthritis. Ann Rheum Dis. 1957;16:494–502.
Xia Y. Magic-angle effect in magnetic resonance imaging of articular cartilage: a review. Invest Radiol. 2000;35(10):602–21.
Joseph GB, McCulloch CE, Nevitt MC, Gersing AS, Schwaiger BJ, Kretzschmar M, et al. Medial femur T2 Z-scores predict the probability of knee structural worsening over 4–8 years: data from the osteoarthritis initiative. J Magn Reson Imaging. 2017;46(4):1128–36.
Li X, Ma C, Link T, Castillo D, Blumenkrantz G, Lozano J, et al. In vivo T1rho and T2 mapping of articular cartilage in osteoarthritis of the knee using 3 Tesla MRI. Osteoarthr Cartil. 2007;15(7):789–97.
Stehling C, Liebl H, Krug R, Lane NE, Nevitt MC, Lynch J, et al. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the osteoarthritis initiative. Radiology. 2010;254(2):509–20.
Mosher TJ, Smith HE, Collins C, Liu Y, Hancy J, Dardzinski BJ, et al. Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology. 2005;234(1):245–9.
Serebrakian AT, Poulos T, Liebl H, Joseph GB, Lai A, Nevitt MC, et al. Weight loss over 48 months is associated with reduced progression of cartilage T2 relaxation time values: data from the osteoarthritis initiative. J Magn Reson Imaging. 2015;41(5):1272–80.
Ferrero G, Sconfienza LM, Fiz F, Fabbro E, Corazza A, Dettore D, et al. Effect of intra-articular injection of intermediate-weight hyaluronic acid on hip and knee cartilage: in-vivo evaluation using T2 mapping. Eur Radiol. 2018;28(6):2345–55.
Welsch GH, Mamisch TC, Zak L, Blanke M, Olk A, Marlovits S, et al. Evaluation of cartilage repair tissue after matrix-associated autologous chondrocyte transplantation using a hyaluronic-based or a collagen-based scaffold with morphological MOCART scoring and biochemical T2 mapping: preliminary results. Am J Sports Med. 2010;38(5):934–42.
Harris S. What’s new for machine learning in medical imaging: predictions for 2019 and beyond. Signify Research. 2018:13. https://s3-eu-west-2.amazonaws.com/signifyresearch/app/uploads/2018/10/16101114/Signify_AI-in-Medical-Imaging-White-Paper.pdf.
Gall R. Machine learning explainability vs interpretability: two concepts that could help restore trust in AI 2018 Available from: https://www.kdnuggets.com/2018/12/machine-learning-explainability-interpretability-ai.html. Accessed Aug 2021.
Onose E. Explainability and auditability in ML: definitions, techniques, and tools 2021 Available from: https://neptune.ai/blog/explainability-auditability-ml-definitions-techniques-tools. Accessed Aug 2021.
Shrikumar A, Greenside P, Kundaje A. Learning important features through propagating activation differences. In: International Conference on Machine Learning. PMLR; 2017. pp. 3145–53.
Springenberg JT, Dosovitskiy A, Brox T, Riedmiller M. Striving for simplicity: the all convolutional net. arXiv preprint arXiv:14126806. 2014.
Linardatos P, Papastefanopoulos V, Kotsiantis S. Explainable AI: a review of machine learning interpretability methods. Entropy. 2021;23(1):18.
Torrey L, Shavlik J. Transfer learning. Handbook of research on machine learning applications. 2009. https://doi.org/10.4018/978-1-60566-766-9.ch011.
Torrey L, Shavlik J, Olivas ES, Guerrero JM, Sober MM, Benedito JM, Lopez AS. Handbook of research on machine learning applications and trends. Hershey: Information Science Reference; 2010. pp. 242–64.
Tiulpin A, Saarakkala S. Automatic grading of individual knee osteoarthritis features in plain radiographs using deep convolutional neural networks. Diagnostics. 2020;10(11):932.
Brownlee J. A gentle introduction to generative adversarial networks (GANs) 2019 Available from: https://machinelearningmastery.com/what-are-generative-adversarial-networks-gans/. Accessed Aug 2021.
Gaj S, Yang M, Nakamura K, Li X. Automated cartilage and meniscus segmentation of knee MRI with conditional generative adversarial networks. Magn Reson Med. 2020;84(1):437–49.
Oneto L, Navarin N, Sperduti A, Anguita D. Recent trends in learning from data: tutorials from the INNS Big Data and Deep Learning Conference (INNSBDDL2019) (Studies in Computational Intelligence) Springer; 2020.
Moustakidis S, Papandrianos NI, Christodolou E, Papageorgiou E, Tsaopoulos D. Dense neural networks in knee osteoarthritis classification: a study on accuracy and fairness. Neural Computing and Applications. 2020.
Funding
This study was funded by NIH R01-AR064771 and NIH R01-AR078917.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Summary statement:
This review article focuses on machine learning (ML) applications for osteoarthritis (OA), with an emphasis on techniques and workflow, followed by applications of deep learning, classical ML, and ensemble ML models for OA and cartilage classification tasks.
Important concepts:
▪ The advancements of artificial intelligence for osteoarthritis applications have been rapid in recent years, particularly innovations of deep learning for image classification, lesion detection, and cartilage segmentation.
▪ Deep learning is a catalyst that can enable cartilage T2 quantification as a short add-on to a routine clinical MR protocol, complementing standard clinical sequences for morphological assessment by providing information on localized cartilage biochemical composition.
▪ The future vision of machine learning applications in clinical radiology hinges on implementation of artificial intelligence for optimizing image protocols, clinical assessment of disease burden, and automatic reporting yielding a faster and more efficient workflow for a radiologist, and a higher level of reproducibility and precision.
▪ By integrating a wide variety of data, from genetics and biochemical serum markers to imaging and clinical factors for a comprehensive assessment of future OA prediction, AI can be used to improve disease prevention as well as identify patients eligible for drug-development clinical trials.
Rights and permissions
About this article
Cite this article
Joseph, G.B., McCulloch, C.E., Sohn, J.H. et al. AI MSK clinical applications: cartilage and osteoarthritis. Skeletal Radiol 51, 331–343 (2022). https://doi.org/10.1007/s00256-021-03909-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-021-03909-2