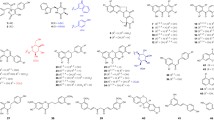
Abstract
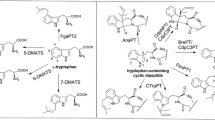
In nature, cyclic dipeptide prenyltransferases catalyze regioselective Friedel-Crafts alkylations of tryptophan-containing cyclic dipeptides. This enzyme class, belonging to the dimethylallyl tryptophan synthase superfamily, is known to be flexible toward aromatic prenyl acceptors, while mostly retaining its typical regioselectivity. Ardeemin fumiquinazoline (FQ) (1), a tryptophan-containing cyclic tripeptide derivative, is assembled in Aspergillus fischeri by the non-ribosomal peptide synthetase ArdA and modified by the prenyltransferase ArdB, leading to the pharmaceutically active hexacyclic ardeemin. Therefore, 1 and its enantiomer ent-ardeemin FQ (2) constitute potential substrates for aromatic prenyltransferases. In this study, we investigated the acceptance of both enantiomers by two cyclic dipeptide C2-prenyltransferases BrePT and FtmPT1 and three C3-prenyltransferases CdpNPT, CdpC3PT, and AnaPT. LC-MS analysis of the incubation mixtures and NMR analysis of the isolated products revealed that the stereochemistry at C11 and C14 in 1 and 2 has a strong influence on their acceptance by these enzymes and the regioselectivity of the prenylation reactions. 1 was very well accepted by BrePT, FtmPT1, and CdpNPT, with C2- or C3-prenylated derivatives as predominant products, which fills the prenylation gaps by tryptophan prenyltransferases reported in a previous study. 2 was a poor substrate for all the enzymes and converted with low regioselectivity and mainly prenylated at C6 and C7 of the indole moiety.



Similar content being viewed by others
References
Alberti F, Foster GD, Bailey AM (2017) Natural products from filamentous fungi and production by heterologous expression. Appl Microbiol Biotechnol 101:493–500
Barrow CJ, Sun HH (1994) Spiroquinazoline, a novel substance P inhibitor with a new carbon skeleton, isolated from Aspergillus flavipies. J Nat Prod 57:471–476
Botta B, Vitali A, Menendez P, Misiti D, Delle MG (2005) Prenylated flavonoids: pharmacology and biotechnology. Curr Med Chem 12:717–739
Chou TC, Depew KM, Zheng YH, Safer ML, Chan D, Helfrich B, Zatorska D, Zatorski A, Bornmann W, Danishefsky SJ (1998) Reversal of anticancer multidrug resistance by the ardeemins. Proc Natl Acad Sci U S A 95:8369–8374
Fan A, Li S-M (2013) One substrate—seven products with different prenylation positions in one-step reactions: prenyltransferases make it possible. Adv Synth Catal 355:2659–2666
Fan A, Winkelblech J, Li S-M (2015) Impacts and perspectives of prenyltransferases of the DMATS superfamily for use in biotechnology. Appl Microbiol Biotechnol 99:7399–7415
Grundmann A, Li S-M (2005) Overproduction, purification and characterization of FtmPT1, a brevianamide F prenyltransferase from Aspergillus fumigatus. Microbiology 151:2199–2207
Haynes SW, Gao X, Tang Y, Walsh CT (2013) Complexity generation in fungal peptidyl alkaloid biosynthesis: a two-enzyme pathway to the hexacyclic MDR export pump inhibitor ardeemin. ACS Chem Biol 8:741–748
Juliano RL, Ling V (1976) A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta 455:152–162
Kartner N, Riordan JR, Ling V (1983) Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science 221:1285–1288
Kato N, Suzuki H, Takagi H, Asami Y, Kakeya H, Uramoto M, Usui T, Takahashi S, Sugimoto Y, Osada H (2009) Identification of cytochrome P450s required for fumitremorgin biosynthesis in Aspergillus fumigatus. Chembiochem 10:920–928
Keller NP, Turner G, Bennett JW (2005) Fungal secondary metabolism—from biochemistry to genomics. Nat Rev Microbiol 3:937–947
Li S-M (2010) Prenylated indole derivatives from fungi: structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat Prod Rep 27:57–78
Liao G, Mai P, Fan J, Zocher G, Stehle T, Li S-M (2018) Complete decoration of the indolyl residue in cyclo-L-Trp-L-Trp with geranyl moieties by using engineered dimethylallyl transferases. Org Lett 20:7201–7205
Liebhold M, Xie X, Li S-M (2013) Breaking cyclic dipeptide prenyltransferase regioselectivity by unnatural alkyl donors. Org Lett 15:3062–3065
Mai P, Zocher G, Ludwig L, Stehle T, Li S-M (2016) Actions of tryptophan prenyltransferases toward fumiquinazolines and their potential application for the generation of prenylated derivatives by combining chemical and chemoenzymatic syntheses. Adv Synth Catal 358:1639–1653
Oya A, Tanaka N, Kusama T, Kim SY, Hayashi S, Kojoma M, Hishida A, Kawahara N, Sakai K, Gonoi T, Kobayashi J (2015) Prenylated benzophenones from Triadenum japonicum. J Nat Prod 78:258–264
Pockrandt D, Li S-M (2013) Geranylation of cyclic dipeptides by the dimethylallyl transferase AnaPT resulting in a shift of prenylation position on the indole ring. Chembiochem 14:2023–2028
Prata-Sena M, Ramos AA, Buttachon S, Castro-Carvalho B, Marques P, Dethoup T, Kijjoa A, Rocha E (2016) Cytotoxic activity of secondary metabolites from marine-derived fungus Neosartorya siamensis in human cancer cells. Phytother Res 30:1862–1871
Resende DISP, Boonpothong P, Sousa E, Kijjoa A, Pinto MMM (2018) Chemistry of the fumiquinazolines and structurally related alkaloids. Nat Prod Rep 36:7–34. https://doi.org/10.1039/C8NP00043C
Ruiz-Sanchis P, Savina SA, Albericio F, Alvarez M (2011) Structure, bioactivity and synthesis of natural products with hexahydropyrrolo[2,3-b]indole. Chemistry 17:1388–1408
Schuller JM, Zocher G, Liebhold M, Xie X, Stahl M, Li S-M, Stehle T (2012) Structure and catalytic mechanism of a cyclic dipeptide prenyltransferase with broad substrate promiscuity. J Mol Biol 422:87–99
Williams RM, Stocking EM, Sanz-Cervera JF (2000) Biosynthesis of prenylated alkaloids derived from tryptophan. Top Curr Chem 209:97–173
Winkelblech J, Fan A, Li S-M (2015) Prenyltransferases as key enzymes in primary and secondary metabolism. Appl Microbiol Biotechnol 99:7379–7397
Wollinsky B, Ludwig L, Hamacher A, Yu X, Kassack MU, Li SM (2012a) Prenylation at the indole ring leads to a significant increase of cytotoxicity of tryptophan-containing cyclic dipeptides. Bioorg Med Chem Lett 22:3866–3869
Wollinsky B, Ludwig L, Xie X, Li S-M (2012b) Breaking the regioselectivity of indole prenyltransferases: identification of regular C3-prenylated hexahydropyrrolo[2,3-b]indoles as side products of the regular C2-prenyltransferase FtmPT1. Org Biomol Chem 10:9262–9270
Woodside AB, Huang Z, Poulter CD (1988) Trisammonium geranyl diphosphate. Org Synth 66:211–215
Yin W-B, Ruan H-L, Westrich L, Grundmann A, Li S-M (2007) CdpNPT, an N-prenyltransferase from Aspergillus fumigatus: overproduction, purification and biochemical characterisation. Chembiochem 8:1154–1161
Yin W-B, Grundmann A, Cheng J, Li S-M (2009) Acetylaszonalenin biosynthesis in Neosartorya fischeri: identification of the biosynthetic gene cluster by genomic mining and functional proof of the genes by biochemical investigation. J Biol Chem 284:100–109
Yin W-B, Yu X, Xie X-L, Li S-M (2010) Preparation of pyrrolo[2,3-b]indoles carrying a ß-configured reverse C3-dimethylallyl moiety by using a recombinant prenyltransferase CdpC3PT. Org Biomol Chem 8:2430–2438
Yin S, Yu X, Wang Q, Liu XQ, Li S-M (2013) Identification of a brevianamide F reverse prenyltransferase BrePT from Aspergillus versicolor with a broad substrate specificity towards tryptophan-containing cyclic dipeptides. Appl Microbiol Biotechnol 97:1649–1660
Yu X, Li S-M (2012) Prenyltransferases of the dimethylallyltryptophan synthase superfamily. Methods Enzymol 516:259–278
Yu X, Liu Y, Xie X, Zheng X-D, Li S-M (2012) Biochemical characterization of indole prenyltransferases: filling the last gap of prenylation positions by a 5-dimethylallyltryptophan synthase from Aspergillus clavatus. J Biol Chem 287:1371–1380
Yu X, Zocher G, Xie X, Liebhold M, Schütz S, Stehle T, Li S-M (2013) Catalytic mechanism of stereospecific formation of cis-configured prenylated pyrroloindoline diketopiperazines by indole prenyltransferases. Chem Biol 20:1492–1501
Acknowledgments
We thank Lena Ludwig-Radtke for the synthesis of DMAPP and Rixa Kraut and Stefan Newel for taking MS and NMR spectra, respectively.
Funding
The Bruker micrOTOF QIII mass spectrometer was financially supported in part by a grant from the Deutsche Forschungsgemeinschaft (INST 160/620-1 to S.-M. L.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This work does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 7028 kb)
Rights and permissions
About this article
Cite this article
Mai, P., Coby, L. & Li, SM. Different behaviors of cyclic dipeptide prenyltransferases toward the tripeptide derivative ardeemin fumiquinazoline and its enantiomer. Appl Microbiol Biotechnol 103, 3773–3781 (2019). https://doi.org/10.1007/s00253-019-09723-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-09723-0