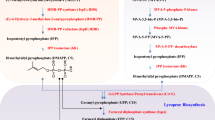
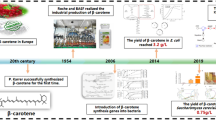
Abstract
Apocarotenoids are natural compounds derived from the oxidative cleavage of carotenoids. Particularly, C13-apocarotenoids are volatile compounds that contribute to the aromas of different flowers and fruits and are highly valued by the Flavor and Fragrance industry. So far, the chemical synthesis of these terpenoids has dominated the industry. Nonetheless, the increasing consumer demand for more natural and sustainable processes raises an interesting opportunity for bio-production alternatives. In this regard, enzymatic biocatalysis and metabolically engineered microorganisms emerge as attractive biotechnological options. The present review summarizes promising bioengineering approaches with regard to chemical production methods for the synthesis of two families of C13-apocarotenoids: ionones/dihydroionones and damascones/damascenone. We discuss each method and its applicability, with a thorough comparative analysis for ionones, focusing on the production process, regulatory aspects, and sustainability.




Similar content being viewed by others
References
Aleu J, Brenna E, Fuganti C, Serra S (1999) Lipase-mediated synthesis of the enantiomeric forms of 4,5-epoxy-4,5-dihydro-α-ionone and 5,6-epoxy-5,6-dihydro-β-ionone. A new direct access to enantiopure (R)- and (S )-α-ionone. J Chem Soc Perkin Trans 1:271–278. doi:10.1039/A808780F
Auldridge ME, Block A, Vogel JT, Dabney-smith C, Mila I, Bouzayen M, Magallanes-Lundback M, Dellapenna D, Mccarty DR, Klee HJ (2006) Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J 45:982–993. doi:10.1111/j.1365-313X.2006.02666.x
Awano K, Ishizaki S, Takazawa O, Kitahara T (2005) Analysis of ambergris tincture. Flavour Frag J 20:18–21. doi:10.1002/ffj.1413
Azzari E, Faggi C, Gelsomini N, Taddei M (1990) Transformation of α- and β-ionones into α- and β-damascone and β-damascenone using allylsilane chemistry. J Org Chem 55:1106–1108. doi:10.1021/jo00290a057
Baldermann S, Kato M, Kurosawa M, Kurobayashi Y, Fujita A, Fleischmann P, Watanabe N (2010) Functional characterization of a carotenoid cleavage dioxygenase 1 and its relation to the carotenoid accumulation and volatile emission during the floral development of Osmanthus fragrans Lour. J Exp Bot 61:2967–2977. doi:10.1093/jxb/erq123
Bauer K, Garbe D, Surburg H (1997) Common fragrance and flavor materials: preparation, properties and uses, 3rd edn. Wiley-VCH, Weinheim
Beekwilder J, van Rossum HM, Koopman F, Sonntag F, Buchhaupt M, Schrader J, Hall RD, Bosch D, Pronk JT, van Maris AJA, Daran JM (2014) Polycistronic expression of a β-carotene biosynthetic pathway in Saccharomyces cerevisiae coupled to β-ionone production. J Biotechnol 192(Pt B):383–392. doi:10.1016/j.jbiotec.2013.12.016
Belin JM, Dumont B, Ropert F (1998) Enzymatic process for the preparation of flavours, in particular the ionones and C6 to C10 aldehydes. Patent US5705372 A
Berger RG (2009) Biotechnology of flavours - the next generation. Biotechnol Lett 31:1651–1659. doi:10.1007/s10529-009-0083-5
Beszant S, Giannini E, Zanoni G, Vidari G (2002) Enantioselective synthesis of both enantiomers of γ-ionone, γ-damascone, karahana lactone and karahana ether. Tetrahedron Asymmetry 13:1245–1255. doi:10.1016/S0957-4166(02)00335-X
Bhosale P, Bernstein PS (2007) Vertebrate and invertebrate carotenoid-binding proteins. Arch Biochem Biophys 458:121–127. doi:10.1016/j.abb.2006.10.005
Bosser A, Belin JM (1994) Synthesis of β-Ionone in an aldehyde/xanthine oxidase/β-carotene system involving free radical formation. Biotechnol Prog 10:129–133. doi:10.1021/bp00026a001
Boulina B, Taranab M, Arreguy-San Miguel B, Delmond B (2007) New preparation methods for α-damascone, γ-damascone, and β-damascenone using pyronenes. Synth Commun 37:2579–2591. doi:10.1080/00397910701462898
Bouvier F, Dogbo O, Camara B (2003) Biosynthesis of the food and cosmetic plant pigment bixin (Annatto). Science 80(300):2089–2091. doi:10.1126/science.1085162
Bovolenta M, Castronovo F, Vadala A, Zanoni G, Vidari G (2004) A simple and efficient highly enantioselective synthesis of α-ionone and α-damascone. J Org Chem 69:8959–8962. doi:10.1021/jo049012j
Brenna E, Fuganti C, Grasselli P, Redaelli M, Serra S (1998) Enzyme-mediated synthesis of (R)- and (S)-α-ionone. J Chem Soc Perkin Trans 1:4129–4134. doi:10.1039/A806943C
Brenna E, Fuganti C, Serra S, Kraft P (2002) Optically active ionones and derivatives : preparation and olfactory properties. Eur J Org Chem 2002:967–978. doi:10.1002/1099-0690(200203)2002:6<967
Brenna E, Fuganti C, Gatti FG, Serra S (2011) Biocatalytic methods for the synthesis of enantioenriched odor active compounds. Chem Rev 111:4036–4072. doi:10.1021/cr100289r
Britton G, Liaaen-Jensen S, Pfander H (2004) Carotenoids handbook, 1st edn. Springer, Basel
Brochado AR, Matos C, Møller BL, Hansen J, Mortensen UH, Patil KR (2010) Improved vanillin production in baker’s yeast through in silico design. Microb Cell Factories 9:1–15. doi:10.1186/1475-2859-9-84
Bruno M, Beyer P, Al-babili S (2015) The potato carotenoid cleavage dioxygenase 4 catalyzes a single cleavage of β-ionone ring-containing carotenes and non-epoxidated xanthophylls. Arch Biochem Biophys 572:126–133. doi:10.1016/j.abb.2015.02.011
Bugoni S, Merlini V, Porta A, Gaillard S, Zanoni G, Nolan SP, Vidari G (2015) Competitive gold-promoted Meyer–Schuster and oxy-cope rearrangements of 3-acyloxy-1,5-enynes: selective catalysis for the synthesis of (+)-(S)-γ-ionone and (−)-(2S,6 R)-cis-γ-irone. Chem Eur J 21:14068–14074. doi:10.1002/chem.201502382
Burdock GA (2009) Fenaroli’s handbook of flavor ingredients, 6th edn. CRC, Boca Raton
Campagnole M, Delmond B (2007) Straightforward synthesis of β-damascenone and β-damascone derivatives from β-ionone via retro-α-ionol. Synth Commun 37:1077–1090. doi:10.1080/00397910701196546
Carlquist M, Gibson B, Yuceer YK, Paraskevopoulou A, Sandell M, Angelov AI, Gotcheva V, Angelov AD, Etschmann M, de Billerbeck GM, Lidén G (2015) Process engineering for bioflavour production with metabolically active yeasts - a mini-review. Yeast 32:123–143. doi:10.1002/yea.3058
Cavalcanti RN, Forster-Carneiro T, Gomes MTMS, Rostagno MA, Prado JM, Meireles MAA (2013) Uses and applications of extracts from natural sources. In: Natural product extraction: principles and applications. Royal Society of Chemistry, Cambridge, pp. 1–57
Cazzonelli CI (2011) Carotenoids in nature: insights from plants and beyond. Funct Plant Biol 38:833–847. doi:10.1071/FP11192
Chapuis C, Jacoby D (2001) Catalysis in the preparation of fragrances and flavours. Appl Catal A Gen 221:93–117. doi:10.1016/S0926-860X(01)00798-0
Chuit P, Bachofen F (1902) Process of making alpha-ionone. Patent US702126 A
Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61:651–679. doi:10.1146/annurev-arplant-042809-112122
Davoli P, Weber RWS (2002) Carotenoid pigments from the red mirror yeast, Sporobolomyces roseus. Mycologist 16:102–108. doi:10.1017/S0269-915X(02)00102-7
Demole E, Enggist P, Säuberli U, Stoll M, Kováts ES (1970) Structure et synthèse de la damascénone (triméthyl-2,6,6-trans-crotonoyl-1-cyclohexadiène-1,3), constituant odorant de l’essence de rose bulgare (Rosa damascena Mill). Helv Chim Acta 53:541–551. doi:10.1002/hlca.19700530310
Díez VK, Marcos BJ, Apesteguía CR, Di Cosimo JI (2009) Ionone synthesis by cyclization of pseudoionone on silica-supported heteropolyacid catalysts. Appl Catal A Gen 358:95–102. doi:10.1016/j.apcata.2009.02.002
Dobler W, Bahr N, Breuer K, Kindler A (2006) Continuous process for producing pseudoionones and ionones. Patent US7141698 B2
Fehr C, Galindo J (1986) Synthesis of (E)-1-propenyl ketones from carboxylic esters and carboxamides by use of mixed organolithium-magnesium reagents. Synthesis of α-damascone, β-damascone, and β-damascenone. Helv Chim Acta 69:228–235. doi:10.1002/hlca.19860690127
Fehr C, Galindo J (1988) Synthesis of (R)-(+)- and (S)-(−)-α-damascone by tandem Grignard reaction-enantioselective protonation: evidence for the intermediacy of a chiral complex. J Am Chem Soc 110:6909–6911. doi:10.1021/ja00228a064
Fehr C, Galindo J (1991) Use of cycloaliphatic ketones as perfuming and flavoring ingredients. Patent US4990496 A
Fehr C, Galindo J (1994) Catalytic enantioselective protonation of enolates. Angew Chem Int Ed 33:1888–1889. doi:10.1002/anie.199418881
Fehr C, Guntern O (1992) Efficient synthesis of enantiomerically pure α-ionone from (R)- and (S)-α-damascone. Helv Chim Acta 75:1023–1028. doi:10.1002/hlca.19920750405
Floss DS, Schliemann W, Schmidt J, Strack D, Walter MH (2008) RNA interference-mediated repression of MtCCD1 in mycorrhizal roots of Medicago truncatula causes accumulation of C27 apocarotenoids, shedding light on the functional role of CCD1. Plant Physiol 148:1267–1282. doi:10.1104/pp.108.125062
Fuganti C, Serra S, Zenoni A (2000) Synthesis and olfactory evaluation of (+)- and (−)-γ-ionone. Helv Chim Acta 83:2761–2768. doi:10.1002/1522-2675(20001004)83:10<2761::AID-HLCA2761>3.0.CO;2-9
Gavrilescu M, Chisti Y (2005) Biotechnology - a sustainable alternative for chemical industry. Biotechnol Adv 23:471–499. doi:10.1016/j.biotechadv.2005.03.004
Gruszecki WI, Strza K (2005) Carotenoids as modulators of lipid membrane physical properties. Biochim Biophys Acta 40:108–115. doi:10.1016/j.bbadis.2004.11.015
Harrison PJ, Bugg TDH (2014) Enzymology of the carotenoid cleavage dioxygenases: reaction mechanisms, inhibition and biochemical roles. Arch Biochem Biophys 544:105–111. doi:10.1016/j.abb.2013.10.005
Hibbert H, Cannon LT (1924) Condensation of citral with ketones and synthesis of some new ionones. J Am Chem Soc 46:119–130. doi:10.1021/ja01666a017
Hirschberg J (2001) Carotenoid biosynthesis in flowering plants. Curr Opin Plant Biol 4:210–218. doi:10.1016/S1369-5266(00)00163-1
Huang FC, Horváth G, Molnár P, Turcsi E, Deli J, Schrader J, Sandmann G, Schmidt H, Schwab W (2009a) Substrate promiscuity of RdCCD1, a carotenoid cleavage oxygenase from Rosa damascena. Phytochemistry 70:457–464. doi:10.1016/j.phytochem.2009.01.020
Huang FC, Molnár P, Schwab W (2009b) Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes. J Exp Bot 60:3011–3022. doi:10.1093/jxb/erp137
Ibdah M, Azulay Y, Portnoy V, Wasserman B, Bar E, Meir A, Burger Y, Hirschberg J, Schaffer AA, Katzir N, Tadmor Y, Lewinsohn E (2006) Functional characterization of CmCCD1, a carotenoid cleavage dioxygenase from melon. Phytochemistry 67:1579–1589. doi:10.1016/j.phytochem.2006.02.009
Isoe S, Katsumura S, Sakan T (1973) The synthesis of damascenone and β-damascone and the possible mechanism of their formation from carotenoids. Helv Chim Acta 56:1514–1516. doi:10.1002/hlca.19730560508
Jäkel C, Paciello R (2010) The asymmetric hydrogenation of enones - access to a new L-menthol synthesis. In: Asymmetric catalysis on industrial scale: challenges, approaches and solutions. Wiley-VCH, Weinheim, pp. 187–205
Kirby J, Keasling JD (2009) Biosynthesis of plant isoprenoids: perspectives for microbial engineering. Annu Rev Plant Biol 60:335–355. doi:10.1146/annurev.arplant.043008.091955
Krings U, Berger RG (1998) Biotechnological production of flavours and fragrances. Appl Microbiol Biotechnol 49:1–8. doi:10.1007/s002530051129
Lalko J, Lapczynski A, McGinty D, Bhatia S, Letizia CS, Api AM (2007a) Fragrance material review on β-ionone. Food Chem Toxicol 45:S241–S247. doi:10.1016/j.fct.2007.09.052
Lalko J, Lapczynski A, Mcginty D, Bhatia S, Letizia CS, Api AM (2007b) Fragrance material review on dihydro-α-ionone. Food Chem Toxicol 45:S221–S224. doi:10.1016/j.fct.2007.09.059
Lalko J, Lapczynski A, McGinty D, Bhatia S, Letizia CS, Api AM (2007c) Fragrance material review on dihydro-β-ionone. Food Chem Toxicol 45:S225–S228. doi:10.1016/j.fct.2007.09.060
Lalko J, Lapczynski A, Politano VT, Mcginty D, Bhatia S, Letizia CS, Api AM (2007d) Fragrance material review on α-ionone. Food Chem Toxicol 45:S235–S240. doi:10.1016/j.fct.2007.09.046
Lapczynski A, Lalko J, Mcginty D, Bhatia S, Letizia CS, Api AM (2007a) Fragrance material review on damascenone. Food Chem Toxicol 45:S172–S178. doi:10.1016/j.fct.2007.09.056
Lapczynski A, Lalko J, Mcginty D, Bhatia S, Letizia CS, Api AM (2007b) Fragrance material review on trans-β-damascone. Food Chem Toxicol 45:S199–S204. doi:10.1016/j.fct.2007.09.015
Lapczynski A, Lalko J, Mcginty D, Bhatia S, Letizia CS, Api AM (2007c) Fragrance material review on α-damascone. Food Chem Toxicol 45:S179–S187. doi:10.1016/j.fct.2007.09.044
Leffingwell and Associates (2015) 2010–2014 Flavor & fragrance industry leaders. http://www.leffingwell.com/top_10.htm. Accessed Dec 2015
Lian J, Si T, Nair NU, Zhao H (2014) Design and construction of acetyl-CoA overproducing Saccharomyces cerevisiae strains. Metab Eng 24:139–149. doi:10.1016/j.ymben.2014.05.010
López J, Essus K, kwon KI, Pereira R, Herzog J, Siewers V, Nielsen J, Agosin E (2015) Production of β-ionone by combined expression of carotenogenic and plant CCD1 genes in Saccharomyces cerevisiae. Microb Cell Factories. doi:10.1186/s12934-015-0273-x
Ly MH, Hoang LC, Belin JM, Waché Y (2008) Improved co-oxidation of β-carotene to β-ionone using xanthine oxidase-generated reactive oxygen species in a multiphasic system. Biotechnol J 3:220–225. doi:10.1002/biot.200700064
Maldonado-Robledo G, Rodríguez-Bustamante E, Sánchez-Contreras A, Rodríguez-Sanoja R, Sánchez S (2003) Production of tobacco aroma from lutein. Specific role of the microorganisms involved in the process. Appl Microbiol Biotechnol 62:484–488. doi:10.1007/s00253-003-1315-6
Messing SAJ, Gabelli SB, Echeverria I, Vogel JT, Guan JC, Tan BC, Klee HJ, McCarty DR, Amzela LM (2010) Structural insights into maize viviparous14, a key enzyme in the biosynthesis of the phytohormone abscisic acid. Plant Cell 22:2970–2980. doi:10.1105/tpc.110.074815
Müller DA (2007) Flavours: the legal framework. In: Flavours and fragrances. Chemistry, bioprocessing and sustainability, 1st edn. Springer, Berlin, pp. 15–24
Nacke C, Hüttmann S, Etschmann MMW, Schrader J (2012) Enzymatic production and in situ separation of natural β-ionone from β-carotene. J Ind Microbiol Biotechnol 39:1771–1778. doi:10.1007/s10295-012-1182-1
Ohloff G, Schade G (1963) Preparation of y-ionone from all-trans-pseudoionone. Angew Chem Int Ed 2:149–150. doi:10.1002/anie.196301493
Oritani T, Yamashita K (1987) Synthesis and absolute stereochemistry of chiral γ-ionone and dihydro-γ-ionone. Agric Biol Chem 51:1271–1275. doi:10.1080/00021369.1987.10868186
Paddon CJ, Westfall PJ, Pitera DJ, Benjamin K, Fisher K, McPhee D, Leavell MD, Tai A, Main A, Eng D, Polichuk DR, Teoh KH, Reed DW, Treynor T, Lenihan J, Jiang H, Fleck M, Bajad S, Dang G, Dengrove D, Diola D, Dorin G, Ellens KW, Fickes S, Galazzo J, Gaucher SP, Geistlinger T, Henry R, Hepp M, Horning T, Iqbal T, Kizer L, Lieu B, Melis D, Moss N, Regentin R, Secrest S, Tsuruta H, Vazquez R, Westblade LF, Xu L, Yu M, Zhang Y, Zhao L, Lievense J, Covello PS, Keasling JD, Reiling KK, Renninger NS, Newman JD (2013) High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 496:528–532. doi:10.1038/nature12051
Pfander H, Semadeni P (1995) The synthesis of optically active enriched (+)-(6R)-α-ionone. Aust J Chem 48:145–151. doi:10.1071/CH9950145
Pickenhagen W (1999) Flavor chemistry - the last 30 years. In: Flavor chemistry. Thirty years of progress. Springer, New York, pp. 75–87
Reyes LH, Gomez JM, Kao KC (2014) Improving carotenoids production in yeast via adaptive laboratory evolution. Metab Eng 21:26–33. doi:10.1016/j.ymben.2013.11.002
Rheude U, Hörcher U, Weller D, Stroezel M (2001) Process for preparing ionones. Patent US6288282 B1
Ristic R, Bindon K, Francis LI, Herderich MJ, Iland PG (2010) Flavonoids and C13-norisoprenoids in Vitis vinifera L. cv. Shiraz: relationships between grape and wine composition, wine colour and wine sensory properties. Aust j Grape Wine R 16:369–388. doi:10.1111/j.1755-0238.2010.00099.x
Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MCY, Withers ST, Shiba Y, Sarpong R, Keasling JD (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440:940–943. doi:10.1038/nature04640
Rodríguez-Bustamante E, Sánchez S (2007) Microbial production of C13-norisoprenoids and other aroma compounds via carotenoid cleavage. Crit Rev Microbiol 33:211–230. doi:10.1080/10408410701473306
Rodríguez-Bustamante E, Gabriela M-R, Ortiz MA, Díaz-Ávalos C, Sánchez S (2005) Bioconversion of lutein using a microbial mixture-maximizing the production of tobacco aroma compounds by manipulation of culture medium. Appl Microbiol Biotechnol 68:174–182. doi:10.1007/s00253-004-1868-z
Rodríguez-Bustamante E, Maldonado-Robledo G, Sánchez-Contreras A, Klimova T, Arreguín-Espinosa R, Sánchez S (2006) Novel method for aroma recovery from the bioconversion of lutein to β-ionone by Trichosporon asahii using a mesoporous silicate material. Appl Microbiol Biotechnol 71:568–573. doi:10.1007/s00253-006-0330-9
Rosati C, Diretto G, Giuliano G (2009) Biosynthesis and engineering of carotenoids and apocarotenoids in plants : state of the art and future prospects. Biotechnol Genet Eng Rev 26:151–174. doi:10.5661/bger-26-139
Royals EE (1946) Cyclization of pseudoionone by acidic reagents. Ind Eng Chem 38:546–548. doi:10.1021/ie50437a028
Rubio A, Santaella M, Gómez MD, Orzaez D, Granell A, Gómez-Gómez L (2008) Cytosolic and plastoglobule-targeted carotenoid dioxygenases from Crocus sativus are both involved in β-ionone release. J Biol Chem 283:24816–24825. doi:10.1074/jbc.M804000200
Sánchez-Contreras A, Jiménez M, Sánchez S (2000) Bioconversion of lutein to products with aroma. Appl Microbiol Biotechnol 54:528–534. doi:10.1007/s002530000421
Sasser DE (1992) Process for the distillative purification of citral. Patent US5094720 A
Saucy G, Marbet R (1967) Über eine neuartige synthese von β-ketoallenen durch reaktion von tertiären acetylencarbinolen mit vinyläthern eine ergiebige methode zur darstellung des pseudojonons und verwandter verbindungen. Helv Chim Acta 50:1158–1167. doi:10.1002/hlca.19670500423
Scalcinati G, Partow S, Siewers V, Schalk M, Daviet L, Nielsen J (2012) Combined metabolic engineering of precursor and co-factor supply to increase α-santalene production by Saccharomyces cerevisiae. Microb Cell Factories. doi:10.1186/1475-2859-11-117
Schaefer B (2014) Natural products in the chemical industry, 2nd edn. Springer, Berlin
Scheibner M, Hülsdau B, Zelena K, Nimtz M, de Boer L, Berger RG, Zorn H (2008) Novel peroxidases of Marasmius scorodonius degrade β-carotene. Appl Microbiol Biotechnol 77:1241–1250. doi:10.1007/s00253-007-1261-9
Schewe H, Mirata MA, Schrader J (2015) Bioprocess engineering for microbial synthesis and conversion of isoprenoids. In: Biotechnology of isoprenoids, 1st edn. Springer, Berlin, pp 251–286
Schulte-Elte KH, Müller BL, Ohloff G (1973) Synthetische übergänge von der jonon- in die damasconreihe. Helv Chim Acta 56:310–320. doi:10.1002/hlca.19730560122
Schwartz SH, Tan BC, Gage DA, Zeevaart JAD, McCarty DR (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 80(276):1872–1874. doi:10.1126/science.276.5320.1872
Schwartz SH, Qin X, Zeevaart JAD (2001) Characterization of a novel carotenoid cleavage dioxygenase from plants. J Biol Chem 276:25208–25211. doi:10.1074/jbc.M102146200
Schwartz SH, Qin X, Loewen MC (2004) The biochemical characterization of two carotenoid cleavage enzymes from Arabidopsis indicates that a carotenoid-derived compound inhibits lateral branching. J Biol Chem 279:46940–46945. doi:10.1074/jbc.M409004200
Sefton MA, Skouroumounis GK, Elsey GM, Taylor DK (2011) Occurrence, sensory impact, formation, and fate of damascenone in grapes, wines, and other foods and beverages. J Agric Food Chem 59:9717–9746. doi:10.1021/jf201450q
Sell CS (2014) Chemistry and the sense of smell, 1st edn. Wiley-VCH, Weinheim
Serra S (2015) Recent advances in the synthesis of carotenoid-derived flavours and fragrances. Molecules 20:12817–12840. doi:10.3390/molecules200712817
Serra S, Fuganti C (2006) Synthesis of the enantiomeric forms of α- and γ-damascone starting from commercial racemic α-ionone. Tetrahedron Asymmetry 17:1573–1580. doi:10.1016/j.tetasy.2006.05.024
Serra S, Fuganti C, Brenna E (2006) Synthesis, olfactory evaluation, and determination of the absolute configuration of the 3,4-didehydroionone stereoisomers. Helv Chim Acta 89:1110–1122. doi:10.1002/hlca.200690109
Serra S, Fuganti C, Brenna E (2007) Two easy photochemical methods for the conversion of commercial ionone alpha into regioisomerically enriched γ-ionone and γ-dihydroionone. Flavour Frag J 22:505–511. doi:10.1002/ffj.1832
Simkin AJ, Underwood BA, Auldridge M, Loucas HM, Shibuya K, Schmelz E, Clark DG, Klee HJ (2004) Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of β-Ionone, a fragrance volatile of petunia flowers. Plant Physiol 136:3504–3514. doi:10.1104/pp.104.049718
Sobotka H, Bloch E, Cahnmann H, Feldbau E, Rosen E (1943) Studies on ionone. II Optical resolution of dl-α-ionone. J Am Chem Soc 65:2061–2062. doi:10.1021/ja01251a008
Soorukram D, Knochel P (2004) Enantioselective synthesis of α-ionone derivatives using an anti SN2‘ substitution of functionalized zinc organometallics. Org Lett 6:2409–2411. doi:10.1021/ol049221q
Steiner K, Ertel H, Tiltscher H (1995) Method for the production of β-ionone from pseudoionone. Patent US5453546 A
Sui X, Kiser PD, Von LJ, Palczewski K (2013) Structural basis of carotenoid cleavage: from bacteria to mammals. Arch Biochem Biophys 539:203–213. doi:10.1016/j.abb.2013.06.012
Surburg H, Panten J (2006) Individual fragrance and flavor materials. In: Common fragrance and flavor materials: preparation, properties and uses. Wiley-VCH, Weinheim, pp. 7–175
Tiemann F (1896) Aromatic ketone and process of making same. Patent US556943 A
Tiemann F, Krüger P (1893) Ueber veilchenaroma. Eur J Inorg Chem 26:2675–2708. doi:10.1002/cber.18930260373
Verwaal R, Wang J, Meijnen JP, Visser H, Sandmann G, van den Berg JA, van Ooyen AJJ (2007) High-level production of beta-carotene in Saccharomyces cerevisiae by successive transformation with carotenogenic genes from Xanthophyllomyces dendrorhous. Appl Env Microbiol 73:4342–4350. doi:10.1128/AEM.02759-06
Verwaal R, Jiang Y, Wang J, Daran J-M, Sandmann G, van den Berg JA, van Ooyen AJJ (2010) Heterologous carotenoid production in Saccharomyces cerevisiae induces the pleiotropic drug resistance stress response. Yeast 27:983–998. doi:10.1002/yea.1807
Vogel JT, Tan BC, Mccarty DR, Klee HJ (2008) The carotenoid cleavage dioxygenase 1 enzyme has broad substrate specificity, cleaving multiple carotenoids at two different bond positions. J Biol Chem 283:11364–11373. doi:10.1074/jbc.M710106200
Waché Y, de Ratuld AB, Belin JM (2006) Dispersion of β-carotene in processes of production of β-ionone by cooxidation using enzyme-generated reactive oxygen species. Process Biochem 41:2337–2341. doi:10.1016/j.procbio.2006.05.010
Wahlberg I (2001) Carotenoid-derived aroma compounds in tobacco. In: Carotenoid-derived aroma compounds. American Chemical Society, Washington, pp. 131–144
Walter MH, Strack D (2011) Carotenoids and their cleavage products: biosynthesis and functions. Nat Prod Rep 28:663–692. doi:10.1039/C0NP00036A
Walter MH, Floss DS, Strack D (2010) Apocarotenoids: hormones, mycorrhizal metabolites and aroma volatiles. Planta 232:1–17. doi:10.1007/s00425-010-1156-3
Waltz E (2015) Engineers of scent. Nat Biotechnol 33:329–332. doi:10.1038/nbt.3191
Wegner G, Kaibel G, Therre J, Aquila W, Fuchs H (2008) Continuous method for producing citral. Patent application WO2008037693 A1
Werkhoff P, Bretschneider W, Güntert M, Hopp R, Surburg H (1991) Chirospecific analysis in flavor and essential oil chemistry part B. Direct enantiomer resolution of trans-α-ionone and trans-α-damascone by inclusion gas chromatography. Eur Food Res Technol 192:111–115. doi:10.1007/BF01202622
Westfall PJ, Pitera DJ, Lenihan JR, Eng D, Woolard FX, Regentin R, Horning T, Tsuruta H, Melis DJ, Owens A, Fickes S, Diolaa D, Benjamin KR, Keasling JD, Leavell MD, McPhee DJ, Renninger NS, Newman JD, Paddon CJ (2012) Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. PNAS 109:E111–E118. doi:10.1073/pnas.1110740109
Winkler JD, Kao KC (2014) Recent advances in the evolutionary engineering of industrial biocatalysts. Genomics 104:406–411. doi:10.1016/j.ygeno.2014.09.006
Winterhalter P, Rouseff RL (2001) Carotenoid-derived aroma compounds. American Chemical Society, Washington
Wu Z, Robinson DS, Hughes RK, Casey R, Hardy D, West SI (1999) Co-oxidation of β-carotene catalyzed by soybean and recombinant pea lipoxygenases. J Agric Food Chem 47:4899–4906. doi:10.1021/jf9901690
Yamamoto T, Yagi K, Ishida K (2009) Method for producing optically active alpha-ionone. US 20090216039 A1
Zelena K, Hardebusch B, Hülsdau B, Berger RG, Zorn H (2009a) Generation of norisoprenoid flavors from carotenoids by fungal peroxidases. J Agric Food Chem 57:9951–9955. doi:10.1021/jf901438m
Zelena K, Zorn H, Nimtz M, Berger RG (2009b) Heterologous expression of the msp2 gene from Marasmius scorodonius. Arch Microbiol 191:397–402. doi:10.1007/s00203-009-0462-2
Zorn H, Langhoff S, Scheibner M, Berger RG (2003a) Cleavage of β,β-carotene to flavor compounds by fungi. Appl Microbiol Biotechnol 62:331–336. doi:10.1007/s00253-003-1309-4
Zorn H, Langhoff S, Scheibner M, Nimtz M, Berger RG (2003b) A peroxidase from Lepista irina cleaves β,β-carotene to flavor compounds. Biol Chem 384:1049–1056. doi:10.1515/BC.2003.117
Acknowledgments
Financial support of FONDECYT grant No. 1130822 for research work on production of bioflavors by metabolic engineering of yeast is greatly acknowledged. Vicente Cataldo and Javiera López acknowledge CONICYT for receiving graduate scholarships for their Ph.D. studies. Vicente Cataldo is also grateful to Pontificia Universidad Católica de Chile for supporting fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Vicente F. Cataldo declares that he has no conflict of interest. Javiera López declares that she has no conflict of interest. Martín Cárcamo declares that he has no conflict of interest. Eduardo Agosin declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Cataldo, V.F., López, J., Cárcamo, M. et al. Chemical vs. biotechnological synthesis of C13-apocarotenoids: current methods, applications and perspectives. Appl Microbiol Biotechnol 100, 5703–5718 (2016). https://doi.org/10.1007/s00253-016-7583-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7583-8