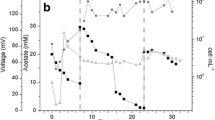
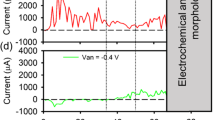
Abstract
In bioelectrochemical systems, exoelectrogenic bacteria respire with anode electrodes as their extracellular electron acceptor; therefore, lower anode potentials can reduce the energy gain to each microbe and select against ones that are not able to respire at a lower potential range. Often fully developed anode communities are compared across bioelectrochemical systems with set anode potentials or fixed external resistances as different operational conditions. However, the comparative effect of the resulting constantly low versus dynamically low anode potentials on the development of anode microbial communities as well as the final cathode microbial communities has not been directly demonstrated. In this study, we used a low fixed anode potential of −250 mV and a higher-current control potential of −119 mV vs. Standard Hydrogen Electrode to approximately correspond with the negative peak anode potential values obtained from microbial fuel cells operated with fixed external resistances of 1 kΩ and 47 Ω, respectively. Pyrosequencing data from a 2-month time series show that a lower set anode potential resulted in a more diverse community than the higher- and variable-potential systems, likely due to the hindered enrichment of a Geobacter-dominated community with limited energy gain at this set potential. In this case, it appears that the selective pressure caused by the low set potential was counteracted by the low energy gain over a 2-month time scale. The air cathode microbial community with constant low anode potentials showed delayed enrichment of denitrifiers or perchlorate-reducing bacteria compared to the fixed external resistance condition.







Similar content being viewed by others
References
Aelterman P, Freguia S, Keller J, Verstraete W, Rabaey K (2008) The anode potential regulates bacterial activity in microbial fuel cells. Appl Microbiol Biotechnol 78:409–418. doi:10.1007/s00253-00713278
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: reevaluation of a unique biological group. Microbiol Mol Biol Rev 43:260–296
Busalmen JP, Esteve-Nunez A, Feliu JM (2008) Whole cell electrochemistry of electricity-producing microorganisms evidence an adaptation for optimal exocellular electron transport. Environ Sci Technol 42:2445–2450. doi:10.1021/es702569y
Cheng S, Liu H, Logan BE (2006) Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem Commun 8:489–494. doi:10.1016/j.elecom.2006.01.010
Cho EJ, Ellington AD (2007) Optimization of the biological component of a bioelectrochemical cell. Bioelectrochemistry 70:165–172. doi:10.1016/j.bioelechem.2006.03.031
Clauwaert P, Verstraete W (2009) Methanogenesis in membraneless microbial electrolysis cells. Appl Microbiol Biotechnol 82:829–836. doi:10.1007/s00253-00817964
Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry 3rd CE, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG (1998) Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544
Commault AS, Lear G, Packer MA, Weld RJ (2013) Influence of anode potentials on selection of Geobacter strains in microbial electrolysis cells. Bioresour Technol 139C:226–234. doi:10.1016/j.biortech.2013.04.047
Dowd SE, Callaway TR, Wolcott RD, Sun Y, McKeehan T, Hagevoort RG, Edrington TS (2008) Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). Bmc Microbiol 8:125. doi:10.1186/1471-2180-8-125
Finkelstein DA, Tender LM, Zeikus JG (2006) Effect of electrode potential on electrode-reducing microbiota. Environ Sci Technol 40:6990–6995. doi:10.1021/es061146m
Harwood CS, Jannasch HW, Canale-Parola E (1982) Anaerobic spirochete from a deep-sea hydrothermal vent. Appl Environ Microbiol 44:234–237
Horn MA, Ihssen J, Matthies C, Schramm A, Acker G, Drake HL (2005) Dechloromonas denitrificans sp. nov., Flavobacterium denitrificans sp. nov., Paenibacillus anaericanus sp. nov and Paenibacillus terrae strain MH72, N2O-producing bacteria isolated from the gut of the earthworm Aporrectodea caliginosa. Int J Syst Evol Microbiol 55:1255–1265. doi:10.1099/ijs.0.634840
Hutchinson AJ, Tokash JC, Logan BE (2011) Analysis of carbon fiber brush loading in anodes on startup and performance of microbial fuel cells. J Power Sources 196:9213–9219. doi:10.1016/j.jpowsour.2011.07.040
Ishak HD, Plowes R, Sen R, Kellner K, Meyer E, Estrada DA, Dowd SE, Mueller UG (2011) Bacterial diversity in Solenopsis invicta and Solenopsis geminata ant colonies characterized by 16S amplicon 454 pyrosequencing. Microb Ecol 61:821–831. doi:10.1007/s00248-01097934
Kiely PD, Cusick R, Call DF, Selembo PA, Regan JM, Logan BE (2011) Anode microbial communities produced by changing from microbial fuel cell to microbial electrolysis cell operation using two different wastewaters. Bioresour Technol 102:388–394. doi:10.1016/j.biortech.2010.05.019
Liu H, Logan BE (2004) Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ Sci Technol 38:4040–4046. doi:10.1021/es0499344
Logan BE (2009) Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol 7:375–381. doi:10.1038/nrmicro2113
Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192. doi:10.1021/es0605016
Lü J, Wu X, Jiang Y, Cai X, Huang L, Yang Y, Wang H, Zeng A, Li A (2014) An extremophile microbacterium strain and its protease production under alkaline conditions. J Basic Microbiol 54:378–385. doi:10.1002/jobm.201200553
Medina RF, Nachappa P, Tamborindeguy C (2011) Differences in bacterial diversity of host-associated populations of Phylloxera notabilis Pergande (Hemiptera: Phylloxeridae) in pecan and water hickory. J Evol Biol 24:761–771. doi:10.1111/j.1420-9101.2010.02215.x
Parot S, Delia ML, Bergel A (2008) Forming electrochemically active biofilms from garden compost under chronoamperometry. Bioresour Technol 99:4809–4816. doi:10.1016/j.biortech.2007.09.047
Quince C, Lanzén A, Curtis TP, Davenport RJ, Hall N, Head IM, Read LF, Sloan WT (2009) Accurate determination of microbial diversity from 454 pyrosequencing data. Nat Methods 6:639–U627. doi:10.1038/nmeth.1361
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi:10.1128/aem.0154109
Schroder U (2007) Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys Chem Chem Phys 9:2619–2629. doi:10.1039/003627m
Shehab N, Li D, Amy G, Logan BE, Saikaly RE (2013) Characterization of bacterial and archaeal communities in air-cathode microbial fuel cells, open circuit and sealed-off reactors. Appl Microbiol Biotechnol 97:9885–9895. doi:10.1007/s00253-013-5025-4
Sun D, Call DF, Kiely PD, Wang AJ, Logan BE (2012) Syntrophic interactions improve power production in formic acid fed MFCs operated with set anode potentials or fixed resistances. Biotechnol Bioeng 109:405–414. doi:10.1002/bit.23348
Torres CI, Krajmalnik-Brown R, Parameswaran P, Marcus AK, Wanger G, Gorby YA, Rittmann BE (2009) Selecting anode-respiring bacteria based on anode potential: phylogenetic, electrochemical, and microscopic characterization. Environ Sci Technol 43:9519–9524. doi:10.1021/es902165y
Vargas IT, Albert IU, Regan JM (2013) Spatial distribution of bacterial communities on volumetric and planar anodes in single-chamber air-cathode microbial fuel cells. Biotechnol Bioeng 110:3059–3062
Wei JC, Liang P, Cao XX, Huang X (2010) A new insight into potential regulation on growth and power generation of Geobacter sulfurreducens in microbial fuel cells based on energy viewpoint. Environ Sci Technol 44:3187–3191. doi:10.1021/es903758m
Wolterink A, Kim S, Muusse M, Kim IS, Roholl PJ, van Ginkel CG, Stams AJ, Kengen SW (2005) Dechloromonas hortensis sp. nov and strain ASK-1, two novel (per)chlorate-reducing bacteria, and taxonomic description of strain GR-1. Int J Syst Evol Microbiol 55:2063–2068. doi:10.1099/ijs.0.63404-0
Yates MD, Kiely PD, Call DF, Rismani-Yazdi H, Bibby K, Peccia J, Regan JM, Logan BE (2012) Convergent development of anodic bacterial communities in microbial fuel cells. ISME J 6:2002–2013. doi:10.1038/ismej.2012.42
Young RA, Mehra V, Sweetser D, Buchanan T, Clarkcurtiss J, Davis RW, Bloom BR (1985) Genes for the major protein antigens of the leprosy parasite mycobacterium-leprae. Nature 316:450–452. doi:10.1038/316450a0
Zhang F, Liu J, Ivanov I, Hatzell MC, Yang W, Ahn Y, Logan BE (2014) Reference and counter electrode positions affect electrochemical characterization of bioanodes in different bioelectrochemical systems. Biotechnol Bioeng. doi:10.1002/bit.25253
Zhang F, Xia X, Luo Y, Sun D, Call DF, Logan BE (2013a) Improving startup performance with carbon mesh anodes in separator electrode assembly microbial fuel cells. Bioresour Technol 133:74–81. doi:10.1016/j.biortech.2013.01.036
Zhang L, Li J, Zhu X, Ye D, Liao Q (2013b) Anodic current distribution in a liter-scale microbial fuel cell with electrode arrays. Chem Eng J 223:623–631. doi:10.1016/j.cej.2013.03.035
Zhu X, Yates MD, Hatzell M, Rao HA, Saikaly RE, Logan BE (2014) Microbial community composition is unaffected by anode potential. Environ Sci Technol 48:1352–1358
Zhu XP, Yates MD, Logan BE (2012) Set potential regulation reveals additional oxidation peaks of Geobacter sulfurreducens anodic biofilms. Electrochem Commun 22:116–119. doi:10.1016/j.elecom.2012.06.013
Acknowledgments
We acknowledge Dr. Justin Tokash’s advice on potentiostatic operation. Financial support under King Abdullah University of Science and Technology (KAUST) KUS-I1-003-13 and Army Research Office Equipment Grant W911NF-11-1-0410 are gratefully acknowledged.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 306 kb)
Rights and permissions
About this article
Cite this article
Yan, H., Yates, M.D. & Regan, J.M. Effects of constant or dynamic low anode potentials on microbial community development in bioelectrochemical systems. Appl Microbiol Biotechnol 99, 9319–9329 (2015). https://doi.org/10.1007/s00253-015-6907-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6907-4