Abstract
Background and objectives
Methotrexate is widely utilized in the chemotherapy of malignant tumors and autoimmune diseases in the pediatric population, but dosing can be challenging. Several population pharmacokinetic models were developed to characterize factors influencing variability and improve individualization of dosing regimens. However, significant covariates included varied across studies. The primary objective of this review was to summarize and discuss population pharmacokinetic models of methotrexate and covariates that influence pharmacokinetic variability in pediatric patients.
Methods
Systematic searches were conducted in the PubMed and EMBASE databases from inception to 7 July 2023. Reporting Quality was evaluated based on a checklist with 31 items. The characteristics of studies and information for model construction and validation were extracted, summarized, and discussed.
Results
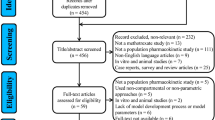
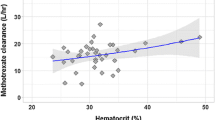
Eighteen studies (four prospective studies and fourteen retrospective studies with sample sizes of 14 to 772 patients and 2.7 to 93.1 samples per patient) were included in this study. Two-compartment models were the commonly used structural models for methotrexate, and the clearance range of methotrexate ranged from 2.32 to 19.03 L/h (median: 6.86 L/h). Body size and renal function were found to significantly affect the clearance of methotrexate for pediatric patients. There were limited reports on the role of other covariates, such as gene polymorphisms and co-medications, in the pharmacokinetic parameters of methotrexate pediatric patients. Internal and external evaluations were used to assess the performance of the population pharmacokinetic models.
Conclusion
A more rigorous external evaluation needs to be performed before routine clinical use to select the appropriate PopPK model. Further research is necessary to incorporate larger cohorts or pool analyses in specific susceptible pediatric populations to improve the understanding of predicted exposure profiles and covariate identification.




Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Pan Z, Yang G, He H, Zhao G, Yuan T, Li Y, Shi W, Gao P, Dong L, Li Y (2016) Concurrent radiotherapy and intrathecal methotrexate for treating leptomeningeal metastasis from solid tumors with adverse prognostic factors: a prospective and single-arm study. Int J Cancer 139(8):1864–1872. https://doi.org/10.1002/ijc.30214
Zhao Z, Hua Z, Luo X, Li Y, Yu L, Li M, Lu C, Zhao T, Liu Y (2022) Application and pharmacological mechanism of methotrexate in rheumatoid arthritis. Biomed Pharmacother = Biomed Pharmacother 150:113074. https://doi.org/10.1016/j.biopha.2022.113074
Sterba J, Valík D, Bajciová V, Kadlecová V, Gregorová V, Mendelová D (2005) High-dose methotrexate and/or leucovorin rescue for the treatment of children with lymphoblastic malignancies: do we really know why, when and how? Neoplasma 52(6):456–463
Mantadakis E, Cole PD, Kamen BA (2005) High-dose methotrexate in acute lymphoblastic leukemia: where is the evidence for its continued use? Pharmacotherapy 25(5):748–755. https://doi.org/10.1592/phco.25.5.748.63584
Moe PJ, Holen A (2000) High-dose methotrexate in childhood all. Pediatr Hematol Oncol 17(8):615–622. https://doi.org/10.1080/08880010050211321
Nguyen HTK, Terao MA, Green DM, Pui CH, Inaba H (2021) Testicular involvement of acute lymphoblastic leukemia in children and adolescents: diagnosis, biology, and management. Cancer 127(17):3067–3081. https://doi.org/10.1002/cncr.33609
Howard SC, McCormick J, Pui CH, Buddington RK, Harvey RD (2016) Preventing and managing toxicities of high-dose methotrexate. Oncologist 21(12):1471–1482. https://doi.org/10.1634/theoncologist.2015-0164
Kawakatsu S, Nikanjam M, Lin M, Le S, Saunders I, Kuo DJ, Capparelli EV (2019) Population pharmacokinetic analysis of high-dose methotrexate in pediatric and adult oncology patients. Cancer Chemother Pharmacol 84(6):1339–1348. https://doi.org/10.1007/s00280-019-03966-4
Panetta JC, Roberts JK, Huang J, Lin T, Daryani VM, Harstead KE, Patel YT, Onar-Thomas A, Campagne O, Ward DA, Broniscer A, Robinson G, Gajjar A, Stewart CF (2020) Pharmacokinetic basis for dosing high-dose methotrexate in infants and young children with malignant brain tumours. Br J Clin Pharmacol 86(2):362–371. https://doi.org/10.1111/bcp.14160
Toksvang LN, Lee SHR, Yang JJ, Schmiegelow K (2022) Maintenance therapy for acute lymphoblastic leukemia: basic science and clinical translations. Leukemia 36(7):1749–1758. https://doi.org/10.1038/s41375-022-01591-4
Gao X, Qian XW, Zhu XH, Yu Y, Miao H, Meng JH, Jiang JY, Wang HS, Zhai XW (2021) Population Pharmacokinetics of High-Dose Methotrexate in Chinese Pediatric patients with Acute Lymphoblastic Leukemia. Front Pharmacol 12. https://doi.org/10.3389/fphar.2021.701452
Hui KH, Chu HM, Fong PS, Cheng WTF, Lam TN (2019) Population Pharmacokinetic Study and Individual Dose adjustments of high-dose methotrexate in Chinese Pediatric patients with Acute Lymphoblastic Leukemia or Osteosarcoma. J Clin Pharmacol 59(4):566–577. https://doi.org/10.1002/jcph.1349
Reiss SN, Buie LW, Adel N, Goldman DA, Devlin SM, Douer D (2016) Hypoalbuminemia is significantly associated with increased clearance time of high dose methotrexate in patients being treated for lymphoma or leukemia. Ann Hematol 95(12):2009–2015. https://doi.org/10.1007/s00277-016-2795-7
McNamara PJ, Alcorn J (2002) Protein binding predictions in infants. AAPS PharmSci 4(1):E4. https://doi.org/10.1208/ps040104
Borsi JD, Sagen E, Romslo I, Moe PJ (1990) Pharmacokinetics and metabolism of methotrexate: an example for the use of clinical pharmacology in pediatric oncology. Pediatr Hematol Oncol 7(1):13–33. https://doi.org/10.3109/08880019009034317
Strawn JR, Poweleit EA, Mills JA, Schroeder HK, Neptune ZA, Specht AM, Farrow JE, Zhang X, Martin LJ, Ramsey LB (2021) Pharmacogenetically guided Escitalopram Treatment for Pediatric anxiety disorders: protocol for a double-blind Randomized Trial. J Personalized Med 11(11). https://doi.org/10.3390/jpm11111188
Wright KD, Panetta JC, Onar-Thomas A, Reddick WE, Patay Z, Qaddoumi I, Broniscer A, Robinson G, Boop FA, Klimo P Jr., Ward D, Gajjar A, Stewart CF (2015) Delayed methotrexate excretion in infants and young children with primary central nervous system tumors and postoperative fluid collections. Cancer Chemother Pharmacol 75(1):27–35. https://doi.org/10.1007/s00280-014-2614-6
Sheiner LB, Rosenberg B, Melmon KL (1972) Modelling of individual pharmacokinetics for computer-aided drug dosage. Comput Biomed Res Int J 5(5):411–459. https://doi.org/10.1016/0010-4809(72)90051-1
Keizer RJ, Ter Heine R, Frymoyer A, Lesko LJ, Mangat R, Goswami S (2018) Model-informed Precision Dosing at the Bedside: Scientific challenges and opportunities. CPT: Pharmacometrics Syst Pharmacol 7(12):785–787. https://doi.org/10.1002/psp4.12353
Duffull SB, Wright DF (2015) What do we learn from repeated population analyses? Br J Clin Pharmacol 79(1):40–47. https://doi.org/10.1111/bcp.12233
Zhang Y, Sun L, Chen X, Zhao L, Wang X, Zhao Z, Mei S (2022) A systematic review of population pharmacokinetic models of methotrexate. Eur J Drug Metab Pharmacokinet 47(2):143–164. https://doi.org/10.1007/s13318-021-00737-6
Wang S, Yin Q, Yang M, Cheng Z, Xie F (2023) External evaluation of population pharmacokinetic models of methotrexate for model-informed precision dosing in pediatric patients with acute lymphoid leukemia. Pharmaceutics 15(2). https://doi.org/10.3390/pharmaceutics15020569
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ (Clinical Res ed) 350:g7647. https://doi.org/10.1136/bmj.g7647
Kanji S, Hayes M, Ling A, Shamseer L, Chant C, Edwards DJ, Edwards S, Ensom MH, Foster DR, Hardy B, Kiser TH, la Porte C, Roberts JA, Shulman R, Walker S, Zelenitsky S, Moher D (2015) Reporting guidelines for clinical pharmacokinetic studies: the ClinPK Statement. Clin Pharmacokinet 54(7):783–795. https://doi.org/10.1007/s40262-015-0236-8
Jamsen KM, McLeay SC, Barras MA, Green B (2014) Reporting a population pharmacokinetic-pharmacodynamic study: a journal’s perspective. Clin Pharmacokinet 53(2):111–122. https://doi.org/10.1007/s40262-013-0114-1
Li ZR, Wang CY, Zhu X, Jiao Z (2021) Population Pharmacokinetics of Levetiracetam: a systematic review. Clin Pharmacokinet 60(3):305–318. https://doi.org/10.1007/s40262-020-00963-2
Rühs H, Becker A, Drescher A, Panetta JC, Pui CH, Relling MV, Jaehde U (2012) Population PK/PD model of homocysteine concentrations after high-dose methotrexate treatment in patients with acute lymphoblastic leukemia. PLoS ONE 7(9):e46015. https://doi.org/10.1371/journal.pone.0046015
Beechinor RJ, Thompson PA, Hwang MF, Vargo RC, Bomgaars LR, Gerhart JG, Dreyer ZE, Gonzalez D (2019) The population pharmacokinetics of high-dose methotrexate in infants with acute lymphoblastic leukemia highlight the need for bedside individualized dose adjustment: a report from the children’s Oncology Group. Clin Pharmacokinet 58(7):899–910. https://doi.org/10.1007/s40262-018-00734-0
Orgel E, Nabais T, Douglas C, Mittelman SD, Neely M (2021) Effect of body fat on population pharmacokinetics of high-dose methotrexate in pediatric patients with acute lymphoblastic leukemia. J Clin Pharmacol 61(6):755–762. https://doi.org/10.1002/jcph.1799
Taylor ZL, Mizuno T, Punt NC, Baskaran B, Navarro Sainz A, Shuman W, Felicelli N, Vinks AA, Heldrup J, Ramsey LB (2020) MTXPK.org: a clinical decision support tool evaluating high-dose methotrexate pharmacokinetics to inform post-infusion care and use of glucarpidase. Clin Pharmacol Ther 108(3):635–643. https://doi.org/10.1002/cpt.1957
Shi ZY, Liu YO, Gu HY, Xu XQ, Yan C, Yang XY, Yan D (2020) Population pharmacokinetics of high-dose methotrexate in Chinese pediatric patients with medulloblastoma. Biopharm Drug Dispos 41(3):101–110. https://doi.org/10.1002/bdd.2221
Zhan M, Sun Y, Zhou F, Wang H, Chen Z, Yan L, Li X (2022) Population pharmacokinetics of methotrexate in paediatric patients with acute lymphoblastic leukaemia and malignant lymphoma. Xenobiotica 52(3):265–273. https://doi.org/10.1080/00498254.2022.2069060
Odoul F, Le Guellec C, Lamagnère JP, Breilh D, Saux MC, Paintaud G, Autret-Leca E (1999) Prediction of methotrexate elimination after high dose infusion in children with acute lymphoblastic leukaemia using a population pharmacokinetic approach. Fundam Clin Pharmacol 13(5):595–604. https://doi.org/10.1111/j.1472-8206.1999.tb00366.x
Aumente D, Buelga DS, Lukas JC, Gomez P, Torres A, García MJ (2006) Population pharmacokinetics of high-dose methotrexate in children with acute lymphoblastic leukaemia. Clin Pharmacokinet 45(12):1227–1238. https://doi.org/10.2165/00003088-200645120-00007
Plard C, Bressolle F, Fakhoury M, Zhang D, Yacouben K, Rieutord A, Jacqz-Aigrain E (2007) A limited sampling strategy to estimate individual pharmacokinetic parameters of methotrexate in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol 60(4):609–620. https://doi.org/10.1007/s00280-006-0394-3
Colom H, Farré R, Soy D, Peraire C, Cendros JM, Pardo N, Torrent M, Domenech J, Mangues MA (2009) Population pharmacokinetics of high-dose methotrexate after intravenous administration in pediatric patients with osteosarcoma. Ther Drug Monit 31(1):76–85. https://doi.org/10.1097/FTD.0b013e3181945624
Buitenkamp TD, Mathôt RA, de Haas V, Pieters R, Zwaan CM (2010) Methotrexate-induced side effects are not due to differences in pharmacokinetics in children with Down syndrome and acute lymphoblastic leukemia. Haematologica 95(7):1106–1113. https://doi.org/10.3324/haematol.2009.019778
Faganel Kotnik B, Grabnar I, Bohanec Grabar P, Dolžan V, Jazbec J (2011) Association of genetic polymorphism in the folate metabolic pathway with methotrexate pharmacokinetics and toxicity in childhood acute lymphoblastic leukaemia and malignant lymphoma. Eur J Clin Pharmacol 67(10):993–1006. https://doi.org/10.1007/s00228-011-1046-z
Jönsson P, Skärby T, Heldrup J, Schrøder H, Höglund P (2011) High dose methotrexate treatment in children with acute lymphoblastic leukaemia may be optimised by a weight-based dose calculation. Pediatr Blood Cancer 57(1):41–46. https://doi.org/10.1002/pbc.22999
Medellin-Garibay SE, Hernández-Villa N, Correa-González LC, Morales-Barragán MN, Valero-Rivera KP, Reséndiz-Galván JE, Ortiz-Zamudio JJ, Milán-Segovia RDC, Romano-Moreno S (2020) Population pharmacokinetics of methotrexate in Mexican pediatric patients with acute lymphoblastic leukemia. Cancer Chemother Pharmacol 85(1):21–31. https://doi.org/10.1007/s00280-019-03977-1
Aarons L, Ogungbenro K (2010) Optimal design of pharmacokinetic studies. Basic Clin Pharmacol Toxicol 106(3):250–255. https://doi.org/10.1111/j.1742-7843.2009.00533.x
Aumente MD, López-Santamaría J, Donoso-Rengifo MC, Reyes-Torres I, Montejano Hervás P (2017) Evaluation of the Novel Methotrexate Architect Chemiluminescent Immunoassay: clinical impact on pharmacokinetic monitoring. Ther Drug Monit 39(5):492–498. https://doi.org/10.1097/ftd.0000000000000434
den Boer E, Koch BC, Huisman R, de Jonge R (2014) Using fluorescence polarization immunoassay for determination of erythrocyte methotrexate polyglutamates, a quick and easy test? Ther Drug Monit 36(6):819–823. https://doi.org/10.1097/ftd.0000000000000085
Mei S, Zhu L, Li X, Wang J, Jiang X, Chen H, Huo J, Yang L, Lin S, Zhao Z (2017) UPLC-MS/MS Analysis of Methotrexate in Human plasma and comparison with the fluorescence polarization immunoassay. Anal Sciences: Int J Japan Soc Anal Chem 33(6):665–670. https://doi.org/10.2116/analsci.33.665
Joerger M, Huitema AD, Illerhaus G, Ferreri AJ (2012) Rational administration schedule for high-dose methotrexate in patients with primary central nervous system lymphoma. Leuk Lymphoma 53(10):1867–1875. https://doi.org/10.3109/10428194.2012.676177
Li J, Gwilt P (2002) The effect of malignant effusions on methotrexate disposition. Cancer Chemother Pharmacol 50(5):373–382. https://doi.org/10.1007/s00280-002-0512-9
Thompson PA, Murry DJ, Rosner GL, Lunagomez S, Blaney SM, Berg SL, Camitta BM, Dreyer ZE, Bomgaars LR (2007) Methotrexate pharmacokinetics in infants with acute lymphoblastic leukemia. Cancer Chemother Pharmacol 59(6):847–853. https://doi.org/10.1007/s00280-006-0388-1
Ince I, Solodenko J, Frechen S, Dallmann A, Niederalt C, Schlender J, Burghaus R, Lippert J, Willmann S (2019) Predictive Pediatric modeling and Simulation using Ontogeny Information. J Clin Pharmacol 59(Suppl 1):S95–s103. https://doi.org/10.1002/jcph.1497
Zhou X, Dun J, Chen X, Xiang B, Dang Y, Cao D (2023) Predicting the correct dose in children: role of computational Pediatric physiological-based pharmacokinetics modeling tools. CPT: Pharmacometrics Syst Pharmacol 12(1):13–26. https://doi.org/10.1002/psp4.12883
Anderson BJ, Holford NH (2008) Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 48:303–332. https://doi.org/10.1146/annurev.pharmtox.48.113006.094708
Millisor VE, Roberts JK, Sun Y, Tang L, Daryani VM, Gregornik D, Cross SJ, Ward D, Pauley JL, Molinelli A, Brennan RC, Stewart CF (2017) Derivation of new equations to estimate glomerular filtration rate in pediatric oncology patients. Pediatr Nephrol 32(9):1575–1584. https://doi.org/10.1007/s00467-017-3693-5
Skärby T, Jönsson P, Hjorth L, Behrentz M, Björk O, Forestier E, Jarfelt M, Lönnerholm G, Höglund P (2003) High-dose methotrexate: on the relationship of methotrexate elimination time vs renal function and serum methotrexate levels in 1164 courses in 264 Swedish children with acute lymphoblastic leukaemia (ALL). Cancer Chemother Pharmacol 51(4):311–320. https://doi.org/10.1007/s00280-002-0552-1
Levey AS, Perrone RD, Madias NE (1988) Serum creatinine and renal function. Annu Rev Med 39:465–490. https://doi.org/10.1146/annurev.me.39.020188.002341
Houlind MB, Petersen KK, Palm H, Jørgensen LM, Aakjær M, Christrup LL, Petersen J, Andersen O, Treldal C (2018) Creatinine-based renal function estimates and dosage of postoperative Pain Management for Elderly Acute hip fracture patients. Pharmaceuticals (Basel Switzerland) 11(3). https://doi.org/10.3390/ph11030088
Schwartz GJ, Feld LG, Langford DJ (1984) A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatr 104(6):849–854. https://doi.org/10.1016/s0022-3476(84)80479-5
Taylor ZL, Vang J, Lopez-Lopez E, Oosterom N, Mikkelsen T, Ramsey LB (2021) Systematic review of pharmacogenetic factors that Influence High-Dose Methotrexate Pharmacokinetics in Pediatric malignancies. Cancers 13(11). https://doi.org/10.3390/cancers13112837
Kodidela S, Suresh Chandra P, Dubashi B (2014) Pharmacogenetics of methotrexate in acute lymphoblastic leukaemia: why still at the bench level? Eur J Clin Pharmacol 70(3):253–260. https://doi.org/10.1007/s00228-013-1623-4
Cheng Y, Wang CY, Li ZR, Pan Y, Liu MB, Jiao Z (2021) Can Population Pharmacokinetics of Antibiotics be extrapolated? Implications of external evaluations. Clin Pharmacokinet 60(1):53–68. https://doi.org/10.1007/s40262-020-00937-4
Donagher J, Martin JH, Barras MA (2017) Individualised medicine: why we need bayesian dosing. Intern Med J 47(5):593–600. https://doi.org/10.1111/imj.13412
Acknowledgements
We thank all authors of the included studies for their hard work.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81673510 and 82073936), Outstanding Scientific Fund of Shengjing Hospital (No. M0779), Natural Science Foundation of Fujian Province (No. 2021J01761), and Joint Funds for the Innovation of Science and Technology of Fujian Province (No. 2021Y9038).
Author information
Authors and Affiliations
Contributions
Y.C. and LM.Z. designed the review and planned the work that led to the manuscript. Y.C., YJ.Z., and Y.Z.performed the literature search and data analysis. Y.C., MB.L., and LM.Z. drafted and revised the manuscript. All authors contributed to the work, approved of the submitted version, and voiced their support for the publishing of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, Y., Zhang, Y., Zhang, Y. et al. Population pharmacokinetic analyses of methotrexate in pediatric patients: a systematic review. Eur J Clin Pharmacol (2024). https://doi.org/10.1007/s00228-024-03665-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00228-024-03665-x