Abstract
Purpose
Nateglinide is commonly used in the treatment of patients with type 2 diabetes mellitus. Our objective was to assess the association between CYP2C9 and SLCO1B1 polymorphisms and the metabolism of nateglinide in healthy Chinese male volunteers.
Methods
A total of 35 healthy Chinese male volunteers with different CYP2C9 and SLCO1B1 genotypes were given a single oral dose of 120 mg nateglinide. Plasma concentrations of nateglinide and blood glucose level were measured up to 8 h.
Results
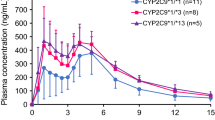
In subjects with the CYP2C9*1/*3 & 521TT, CYP2C9*1/*1 & 521TC/CC and CYP2C9*1/*3 & 521TC genotype, AUC0-∞ of nateglinide was 56 %, 34 % and 56 % higher (P = 0.002, P = 0.041 and P = 0.013, respectively), and the CL/F of nateglinide was 35 %, 11 % and 36 % lower (P = 0.000, P = 0.003 and P = 0.002, respectively) than that in the reference group. When only considering 521 T>C polymorphism, it had no significant association with the pharmacokinetics of nateglinide. CYP2C9*3 and 521 T>C polymorphisms were the significant predictors of the AUC0-∞ and CL/F of nateglinide (adjusted multiple R 2 = 34 % and 43 %, respectively) according to multiple linear regression analyses, but they have no significant association with changes in the blood glucose-lowering effect of nateglinide.
Conclusions
Both SLCO1B1 521 T>C and the CYP2C9*3 polymorphisms can significantly affect the pharmacokinetics of nateglinide, but they could only partially explain the interindividual variability of plasma concentration of nateglinide. Moreover, 521 T>C and the CYP2C9*3 polymorphisms have no effect on pharmacodynamics of nateglinide in healthy Chinese male subjects.


Similar content being viewed by others
References
Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q, Zhu D, Ge J, Lin L, Chen L, Guo X, Zhao Z, Li Q, Zhou Z, Shan G, He J (2010) Prevalence of diabetes among men and women in China. N Engl J Med 362(12):1090–1101
McLeod JF (2004) Clinical pharmacokinetics of nateglinide: a rapidly-absorbed, short-acting insulinotropic agent. Clin Pharmacokinet 43(2):97–120
Kalbag JB, Walter YH, Nedelman JR, McLeod JF (2001) Mealtime glucose regulation with nateglinide in healthy volunteers: comparison with repaglinide and placebo. Diabetes Care 24(1):73–77
Weaver ML, Orwig BA, Rodriguez LC, Graham ED, Chin JA, Shapiro MJ, McLeod JF, Mangold JB (2001) Pharmacokinetics and metabolism of nateglinide in humans. Drug Metab Dispos 29(4 Pt 1):415–421
Sullivan-Klose TH, Ghanayem BI, Bell DA, Zhang ZY, Kaminsky LS, Shenfield GM, Miners JO, Birkett DJ, Goldstein JA (1996) The role of the CYP2C9-Leu359 allelic variant in the tolbutamide polymorphism. Pharmacogenetics 6(4):341–349
Kirchheiner J, Meineke I, Muller G, Bauer S, Rohde W, Meisel C, Roots I, Brockmoller J (2004) Influence of CYP2C9 and CYP2D6 polymorphisms on the pharmacokinetics of nateglinide in genotyped healthy volunteers. Clin Pharmacokinet 43(4):267–278
Abe T, Kakyo M, Tokui T, Nakagomi R, Nishio T, Nakai D, Nomura H, Unno M, Suzuki M, Naitoh T, Matsuno S, Yawo H (1999) Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1. J Biol Chem 274(24):17159–17163
Hsiang B, Zhu Y, Wang Z, Wu Y, Sasseville V, Yang WP, Kirchgessner TG (1999) A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J Biol Chem 274(52):37161–37168
Tirona RG, Leake BF, Merino G, Kim RB (2001) Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J Biol Chem 276(38):35669–35675
Nozawa T, Nakajima M, Tamai I, Noda K, Nezu J, Sai Y, Tsuji A, Yokoi T (2002) Genetic polymorphisms of human organic anion transporters OATP-C (SLC21A6) and OATP-B (SLC21A9): allele frequencies in the Japanese population and functional analysis. J Pharmacol Exp Ther 302(2):804–813
Kameyama Y, Yamashita K, Kobayashi K, Hosokawa M, Chiba K (2005) Functional characterization of SLCO1B1 (OATP-C) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15 + C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet Genomics 15(7):513–522
Zhang W, He YJ, Han CT, Liu ZQ, Li Q, Fan L, Tan ZR, Zhang WX, Yu BN, Wang D, Hu DL, Zhou HH (2006) Effect of SLCO1B1 genetic polymorphism on the pharmacokinetics of nateglinide. Br J Clin Pharmacol 62(5):567–572
Kalliokoski A, Neuvonen M, Neuvonen PJ, Niemi M (2008) Different effects of SLCO1B1 polymorphism on the pharmacokinetics and pharmacodynamics of repaglinide and nateglinide. J Clin Pharmacol 48(3):311–321
Niemi M (2007) Role of OATP transporters in the disposition of drugs. Pharmacogenomics 8(7):787–802
Maeda K, Ieiri I, Yasuda K, Fujino A, Fujiwara H, Otsubo K, Hirano M, Watanabe T, Kitamura Y, Kusuhara H, Sugiyama Y (2006) Effects of organic anion transporting polypeptide 1B1 haplotype on pharmacokinetics of pravastatin, valsartan, and temocapril. Clin Pharmacol Ther 79(5):427–439
Wen J, Xiong Y (2010) OATP1B1 388A>G polymorphism and pharmacokinetics of pitavastatin in Chinese healthy volunteers. J Clin Pharm Ther 35(1):99–104
Kalliokoski A, Backman JT, Neuvonen PJ, Niemi M (2008) Effects of the SLCO1B1*1B haplotype on the pharmacokinetics and pharmacodynamics of repaglinide and nateglinide. Pharmacogenet Genomics 18(11):937–942
Nasu K, Kubota T, Ishizaki T (1997) Genetic analysis of CYP2C9 polymorphism in a Japanese population. Pharmacogenetics 7(5):405–409
Huang MJ, Kua KE, Teng HC, Tang KS, Weng HW, Huang CS (2004) Risk factors for severe hyperbilirubinemia in neonates. Pediatr Res 56(5):682–689
Niemi M, Pasanen MK, Neuvonen PJ (2006) SLCO1B1 polymorphism and sex affect the pharmacokinetics of pravastatin but not fluvastatin. Clin Pharmacol Ther 80(4):356–366
Pasanen MK, Fredrikson H, Neuvonen PJ, Niemi M (2007) Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther 82(6):726–733
Du J, Yu L, Wang L, Zhang A, Shu A, Xu L, Xu M, Shi Y, Li X, Feng G, Xing Q, He L (2007) Differences in CYP3A41G genotype distribution and haplotypes of CYP3A4, CYP3A5 and CYP3A7 in 3 Chinese populations. Clin Chim Acta 383(1–2):172–174
Gao Y, Zhang LR, Fu Q (2008) CYP3A4*1G polymorphism is associated with lipid-lowering efficacy of atorvastatin but not of simvastatin. Eur J Clin Pharmacol 64(9):877–882
Zhang W, Chang YZ, Kan QC, Zhang LR, Li ZS, Lu H, Wang ZY, Chu QJ, Zhang J (2010) CYP3A4*1G genetic polymorphism influences CYP3A activity and response to fentanyl in Chinese gynecologic patients. Eur J Clin Pharmacol 66(1):61–66
Yuan R, Zhang X, Deng Q, Wu Y, Xiang G (2011) Impact of CYP3A4*1G polymorphism on metabolism of fentanyl in Chinese patients undergoing lower abdominal surgery. Clin Chim Acta 412(9–10):755–760
Miller WL (2005) Minireview: regulation of steroidogenesis by electron transfer. Endocrinology 146(6):2544–2550
Agrawal V, Huang N, Miller WL (2008) Pharmacogenetics of P450 oxidoreductase: effect of sequence variants on activities of CYP1A2 and CYP2C19. Pharmacogenet Genomics 18(7):569–576
Oneda B, Crettol S, Jaquenoud Sirot E, Bochud M, Ansermot N, Eap CB (2009) The P450 oxidoreductase genotype is associated with CYP3A activity in vivo as measured by the midazolam phenotyping test. Pharmacogenet Genomics 19(11):877–883
Yang G, Fu Z, Chen X, Yuan H, Yang H, Huang Y, Ouyang D, Tan Z, Tan H, Huang Z, Zhou H (2011) Effects of the CYP oxidoreductase Ala503Val polymorphism on CYP3A activity in vivo: a randomized, open-label, crossover study in healthy Chinese men. Clin Ther 33(12):2060–2070
Acknowledgments
This work was supported by Hunan Provincial Innovation Foundation for Postgraduates of China (No. 2340-74236000007), National Natural Science Foundation of China (No. 81072706).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, Y., Wang, G., Zhang, W. et al. Effect of CYP2C9 and SLCO1B1 polymorphisms on the pharmacokinetics and pharmacodynamics of nateglinide in healthy Chinese male volunteers. Eur J Clin Pharmacol 69, 407–413 (2013). https://doi.org/10.1007/s00228-012-1364-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1364-9