Abstract
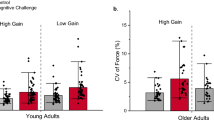
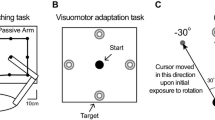
The purpose of this study was to determine whether magnified visual feedback during position-holding contractions exacerbates the age-associated differences in motor output variability due to changes in the neural activation of the agonist muscle in the upper and lower limb. Twelve young (18–35 years) and ten older adults (65–85 years) were instructed to accurately match a target position at 5° of index finger abduction and ankle dorsiflexion while lifting 10 % of their 1 repetition maximum (1RM) load. Position was maintained at three different visual angles (0.1°, 1°, and 4°) that varied across trials. Each trial lasted 25 s and visual feedback of position was removed from 15 to 25 s. Positional error was quantified as the root mean square error (RMSE) of the subject’s performance from the target. Positional variability was quantified as the standard deviation of the position data. The neural activation of the first dorsal interosseus and tibialis anterior was measured with surface electromyography (EMG). Older adults were less accurate compared with young adults and the RMSE decreased significantly with an increase in visual gain. As expected, and independent of limb, older adults exhibited significantly greater positional variability compared with young adults that was exacerbated with magnification of visual feedback (1° and 4°). This increase in variability at the highest magnification of visual feedback was predicted by a decrease in power from 12 to 30 Hz of the agonist EMG signal. These findings demonstrate that motor control in older adults is impaired by magnified visual feedback during positional tasks.






Similar content being viewed by others
References
Addison PS (2002) The illustrated wavelet transform handbook. Taylor & Francis Group, New York
Baudry S, Maerz AH, Enoka RM (2010) Presynaptic modulation of Ia afferents in young and old adults when performing force and position control. J Neurophysiol 103:623–631
Baweja HS, Patel BK, Martinkewiz JD, Vu J, Christou EA (2009) Removal of visual feedback alters muscle activity and reduces force variability during constant isometric contractions. Exp Brain Res 197:35–47
Baweja HS, Kennedy DM, Vu JL, Vaillancourt DE, Christou EA (2010a) Greater amount of visual feedback decreases force variability by reducing force oscillations from 0–1 and 3–7 Hz. Eur J Appl Physiol 108:935–943
Baweja HS, Kwon MH, Glover SQ, Christou EA (2010b) Greater amount of visual feedback decreases error but not variability during movements and positional tasks with the finger and foot. Society for Neuroscience, San Diego
Chao EYS, An KN, Cooney WP, Linschied RL (1989) Biomechanics of the hand. A basic research study. World Scientific Publishing, Teaneack
Christou EA (2010) Aging and variability of voluntary contractions. Exerc Sport Sci Rev 39:77–84
Christou EA, Carlton LG (2002) Age and contraction type influence motor output variability in rapid discrete tasks. J Appl Physiol 93:489–498
Christou EA, Enoka RM (2010) Aging and movement errors when lifting and lowering light loads. Age (Dordr) 33:393–407
Christou EA, Jakobi JM, Critchlow A, Fleshner M, Enoka RM (2004) The 1- to 2-Hz oscillations in muscle force are exacerbated by stress, especially in older adults. J Appl Physiol 97:225–235
Coombes SA, Corcos DM, Sprute L, Vaillancourt DE (2010) Selective regions of the visuomotor system are related to gain-induced changes in force error. J Neurophysiol 103:2114–2123
Green SB, Salkind NJ (2002) Using SPSS for the windows and macintosh: analyzing and understanding data. Prentice Hall, Upper Saddle River, NJ
Griffith EE, Yoon T, Hunter SK (2010) Age and load compliance alter time to task failure for a submaximal fatiguing contraction with the lower leg. J Appl Physiol 108:1510–1519
Homma T, Sakai T (1991) Ramification pattern of intermetacarpal branches of the deep branch (ramus profundus) of the ulnar nerve in the human hand. Acta Anat (Basel) 141:139–144
Hunter SK, Ryan DL, Ortega JD, Enoka RM (2002) Task differences with the same load torque alter the endurance time of submaximal fatiguing contractions in humans. J Neurophysiol 88:3087–3096
Kennedy DM, Christou EA (2011) Greater amount of visual information exacerbates force control in older adults during constant isometric contractions. Exp Brain Res 213:351–361
Kwon MH, Baweja HS, Christou EA (2011) Age-associated differences in positional variability are greater with the lower limb. J Mot Behav 43:357–360
Nafati G, Schmied A, Rossi-Durand C (2005) Changes in the inhibitory control exerted by the antagonist Ia afferents on human wrist extensor motor units during an attention-demanding motor task. J Neurophysiol 93:2350–2353
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Prodoehl J, Vaillancourt DE (2010) Effects of visual gain on force control at the elbow and ankle. Exp Brain Res 200:67–79
Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD (1997) Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex 7:268–282
Slifkin AB, Vaillancourt DE, Newell KM (2000) Intermittency in the control of continuous force production. J Neurophysiol 84:1708–1718
Sosnoff JJ, Newell KM (2006) Information processing limitations with aging in the visual scaling of isometric force. Exp Brain Res 170:423–432
Sosnoff JJ, Vaillancourt DE, Newell KM (2004) Aging and rhythmical force output: loss of adaptive control of multiple neural oscillators. J Neurophysiol 91:172–181
Torrence C, Compo GP (1998) A practical guide to wavelet analysis. Bull Amer Meteor Soc 79:61–78
Tracy BL (2007a) Force control is impaired in the ankle plantarflexors of elderly adults. Eur J Appl Physiol 101:629–636
Tracy BL (2007b) Visuomotor contribution to force variability in the plantarflexor and dorsiflexor muscles. Hum Mov Sci 26:796–807
Tracy BL, Dinenno DV, Jorgensen B, Welsh SJ (2007) Aging, visuomotor correction, and force fluctuations in large muscles. Med Sci Sports Exerc 39:469–479
Vaillancourt DE, Slifkin AB, Newell KM (2002) Inter-digit individuation and force variability in the precision grip of young, elderly, and Parkinson’s disease participants. Mot Control 6:113–128
Vaillancourt DE, Larsson L, Newell KM (2003) Effects of aging on force variability, single motor unit discharge patterns, and the structure of 10, 20, and 40 Hz EMG activity. Neurobiol Aging 24:25–35
Vaillancourt DE, Haibach PS, Newell KM (2006) Visual angle is the critical variable mediating gain-related effects in manual control. Exp Brain Res 173:742–750
van der Lubbe RH, Verleger R (2002) Aging and the Simon task. Psychophysiology 39:100–110
Webber SC, Porter MM (2010) Effects of ankle power training on movement time in mobility-impaired older women. Med Sci Sports Exerc 42:1233–1240
Zazula D, Karlsson S, Doncarli C (2004) In: Merletti R (ed) Elelctromyography: physiology, engineering, and noninvasive applications. Wiley, Hoboken, pp 259–304
Acknowledgments
We would like to thank Jonathan Leake for computer programming, Deanna M. Kennedy and Shauna Q. Glover for subject recruitment. This study was supported by NIH R01 AG031769 to Evangelos A. Christou.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baweja, H.S., Kwon, M. & Christou, E.A. Magnified visual feedback exacerbates positional variability in older adults due to altered modulation of the primary agonist muscle. Exp Brain Res 222, 355–364 (2012). https://doi.org/10.1007/s00221-012-3219-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-012-3219-0