Abstract
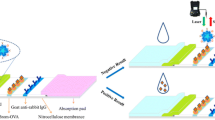
Clenbuterol is a β-adrenergic receptor agonist that can be used as a bronchodilator for the treatment of respiratory diseases or as an additive feed in livestock to improve conversion rate of lean meat. However, the residue of clenbuterol in food can be a threat to human health. Therefore, it is important to monitor clenbuterol in foods. In this paper, a fluorescence inner filtration immunoassay (FIFI) method for the detection of clenbuterol was developed based on the principle of immunoreactive competitive method and the theory of fluorescence quenching, which was constructed from a fluorescence-quenching detection system and a separation system of magnetic immunization. In the magnetic immunization separation system, the detection antigen competes with the biotin-labeled antigen for binding to colloidal gold-labeled clenbuterol antibody and the immunocomplex is enriched by streptavidin- and biotin-specific reactions on the surface of magnetic beads. The remaining complex as a fluorescence quencher in the detection system after centrifugation, and rhodamine 6G acts as a fluorescence agent. The quantitative detection of clenbuterol was accomplished by achieving fluorescence quenching under excitation light irradiation. The standard solutions of clenbuterol in the range of 0.03–5.0 ng/mL exhibited good correlation (r = 0.9996) with the fluorescence intensity, with limit of detection 0.01 ng/mL and recoveries ranging from 98.1 to 101.2%. Moreover, there was no significant difference between the results obtained after testing valid samples by FIFI and ELISA. Therefore, our developed method is sensitive and efficient, simple to operate, and has great potential for on-site detection of clenbuterol.





Similar content being viewed by others
References
Knych HK, Harrison LM, Steinmetz SJ, Chouicha N, Kass PH (2016) Differential expression of skeletal muscle genes following administration of clenbuterol to exercised horses. BMC Genom 17:596
Al-Majed AA, Khalil NY, Khbrani I, Abdel-Aziz HA (2017) Clenbuterol hydrochloride profiles. Drug Subst Excip Relat Methodol 42:91–123
Kintz P, Gheddar L, Ameline A, Dumestre-Toulet V, Verschoore M, Comte J, Raul JS (2019) Complete post-mortem investigations in a death involving clenbuterol after long-term abuse. J Anal Toxicol 43:660–665
Espinoza W, Vargas Jentzsch P, Gualpa F, Andrade P, Moreno C, Vaca I, Betancourt R, Medina L, Enriquez D, Guijarro M, Garrido P, Bravo J, Ulic S, Montalvo Garcia G, Ortega F, Stolker L, Ramos L (2020) Survey of clenbuterol in bovine muscle and liver in Ecuador. Food Addit Contam Part B Surveill 13:107–114
Žlak N, Košuta D, Potisek M, Stevanović Ž (2017) Clenbuterol toxicity in a young male athlete. Toxin Rev 37:182–186
Solheim SA, Jessen S, Morkeberg J, Thevis M, Dehnes Y, Eibye K, Hostrup M, Nordsborg NB (2020) Single-dose administration of clenbuterol is detectable in dried blood spots. Drug Test Anal 12:1366–1372
Huang Z, Xiong Z, Chen Y, Hu S, Lai W (2019) Sensitive and matrix-tolerant lateral flow immunoassay based on fluorescent magnetic nanobeads for the detection of clenbuterol in swine urine. J Agric Food Chem 67:3028–3036
Cong Y, Dong H, Wei XY, Zhang LQ, Bai JK, Wu JL, Huang JX, Gao ZQ, Ueda H, Dong JH (2019) A novel murine antibody and an open sandwich immunoassay for the detection of clenbuterol. Ecotox Environ Safe 182:109473
Ma LY, Nilghaz A, Choi JR, Liu XQ, Lu XN (2018) Rapid detection of clenbuterol in milk using microfluidic paper-based ELISA. Food Chem 246:437–441
Cheng J, Wang S, Zhang S, Wang PL, Xie JC, Han CQ, Su XO (2019) Rapid and sensitive determination of clenbuterol residues in animal urine by surface-enhanced Raman spectroscopy. Sens Actuator B Chem 279:7–14
Meza-Márquez OG, Gallardo-Velázquez T, Osorio-Revilla G, Dorantes-Álvarez L (2012) Detection of clenbuterol in beef meat, liver and kidney by mid-infrared spectroscopy (FT-Mid IR) and multivariate analysis. Int J Food Sci Technol 47:2342–2351
Sun Y, Chen H, Ma P, Li JY, Zhang Z, Shi H, Zhang XD (2020) In situ synthesis of graphene oxide/gold nanocomposites as ultrasensitive surface-enhanced Raman scattering substrates for clenbuterol detection. Anal Bioanal Chem 412:193–201
Yu M, Hu YJ, Liu JZ (2017) Simultaneous detection of clenbuterol and ractopamine based on multiplexed competitive surface enhanced Raman scattering (SERS) immunoassay. New J Chem 41:10407–10414
Kang JY, Zhang YJ, Li X, Miao LJ, Wu AG (2016) A rapid colorimetric sensor of clenbuterol based on cysteamine-modified gold nanoparticles. ACS Appl Mater Interfaces 8:1–5
Simon T, Shellaiah M, Steffi P, Sun KW, Ko FH (2018) Development of extremely stable dual functionalized gold nanoparticles for effective colorimetric detection of clenbuterol and ractopamine in human urine samples. Anal Chim Acta 1023:96–104
Panneerselvam RL, Liu GK, Wang YH, Liu JY, Ding SY, Li JF, Wu DY, Tian ZQ (2017) Surface-enhanced Raman spectroscopy: bottlenecks and future directions. Chem Commun (Camb) 54:10–25
Zhang ZH, Duan FH, He LH, Peng DL, Yan FF, Wang MH, Zong W, Jia CX (2016) Electrochemical clenbuterol immunosensor based on a gold electrode modified with zinc sulfide quantum dots and polyaniline. Microchim Acta 183:1089–1097
Guo RX, Xu Q, Wang DY, Hu XY (2007) Trace determination of clenbuterol with an MWCNT-Nafion nanocomposite modified electrode. Microchim Acta 161:265–272
Ge Y, Camarada MB, Xu LJ, Qu MR, Liang H, Zhao EL, Li MF, Wen YP (2018) A highly stable black phosphorene nanocomposite for voltammetric detection of clenbuterol. Microchim Acta 185:566
Huang Q, Zhang WT, Yan LZ, Zhang MY, Yang QF, Huang LJ, Yang BW, Hu N, Suo YR, Wang JL, Zhang DH (2018) An improved clenbuterol detection by immunochromatographic assay with bacteria@Au composite as signal amplifier. Food Chem 262:48–55
Guo PQ, Luo ZM, Xu XY, Zhou YL, Zhang BL, Chang RM et al (2017) Development of molecular imprinted column-on line-two dimensional liquid chromatography for selective determination of clenbuterol residues in biological samples. Food Chem 217:628–636
Yan KP, Zhang HQ, Hui WL, Zhu HL, Li XB, Zhong FY, Tong XE, Chen C (2016) Rapid screening of toxic salbutamol, ractopamine, and clenbuterol in pork sample by high-performance liquid chromatography-UV method. J Food Drug Anal 24:277–283
Liu HB, Gan N, Chen YJ, Ding QQ, Huang J, Lin SC, Cao YT, Li TH (2016) Novel method for the rapid and specific extraction of multiple β2-agonist residues in food by tailor-made Monolith-MIPs extraction disks and detection by gas chromatography with mass spectrometry. J Sep Sci 39:3578–3585
Cheng J, Wang S, Su XO (2013) Simultaneous identification and quantification of 20 β-receptor agonists in feed using gas chromatography-tandem mass spectrometry. PLoS ONE 8:e76400
Wang LQ, Zeng ZL, Wang Z, Guo JY, He LM (2015) Influence of water in samples on residues analysis of beta-agonists in porcine tissues and urine using liquid chromatography tandem mass spectrometry. Food Anal Methods 9:1904–1911
Montes Nino AM, Granja RH, Reche KV, Giannotti FM, de Souza JK, Ferrari SP, Dos Santos AD, Wanschel AC, Salerno AG (2017) Laboratory validation of an LC-MS/MS method for the detection of ractopamine, clenbuterol and salbutamol in bovine and swine muscle at sub-mug/kg regulatory limit. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 34:785–792
Bao Y, Yang F, Yang XR (2012) Capillary electrophoresis coupled with electrochemiluminescence for the facile separation and determination of salbutamol and clenbuterol in urine. Electroanalysis 24:1597–1603
Lu YF, Wang H, Xue Y, Gu X, Wang Y, Yan C (2015) Preparation and evaluation of monodispersed, submicron, non-porous silica particles functionalized with β-CD derivatives for chiral-pressurized capillary electrochromatography. Electrophoresis 36:2120–2127
Cheng J, Su XO, Wang S, Zhao YP (2016) Highly sensitive detection of clenbuterol in animal urine using immunomagnetic bead treatment and surface-enhanced Raman spectroscopy. Sci Rep 6:32637
Fu QQ, Liang JJ, Lan CF, Zhou KN, Shi CY, Tang Y (2014) Development of a novel dual-functional lateral-flow sensor for on-site detection of small molecule analytes. Sens Actuator B Chem 203:683–689
Chen XJ, Xu YY, Yu JS, Li JT, Zhou XL, Wu CY, Ji QL, Ren Y, Wang LQ, Huang ZY, Zhuang HL, Piao L, Head R, Wang YJ, Lou JT (2014) Antigen detection based on background fluorescence quenching immunochromatographic assay. Anal Chim Acta 841:44–50
Shan S, Lai WH, Xiong YH, Wei H, Xu HY (2015) Novel strategies to enhance lateral flow immunoassay sensitivity for detecting foodborne pathogens. J Agric Food Chem 63:745–753
Zhang YY, Li M, Cui Y, Hong X, Du DL (2018) Using of tyramine signal amplification to improve the sensitivity of ELISA for aflatoxin B1 in edible oil samples. Food Anal Methods 11:2553–2560
Hu CM, Ma L, Guan M, Mi F, Peng F, Guo C, Sun SJ, Wang XM, Liu TW, Li JT (2020) SERS-based magnetic immunoassay for simultaneous detection of cTnI and H-FABP using core-shell nanotags. Anal Methods 12:5442–5449
Frens G (1973) Controlled nucleation for regulation of particle size in monodisperse gold suspensions. Nat Phys Sci 241:20–22
The State Food and Drug Administration. Guidelines for the performance evaluation of in vitro diagnostic reagents (Draft). 2011-03-24
Duan N, Gong WH, Wu SJ, Wang ZP (2017) Selection and application of ssDNA aptamers against clenbuterol hydrochloride based on ssDNA library immobilized SELEX. J Agric Food Chem 65:1771–1777
Qu XL, Lin H, Du SY, Sui JX, Zhang XL, Cao LM (2016) Development of a nano-gold capillary immunochromatographic assay for rapid and semi-quantitative detection of clenbuterol residues. Food Anal Methods 9:2531–2540
Peng DP, Zhang LY, Chen ST, Pan YH, Tao YF, Wang YL, Yuan ZH (2017) Development of monoclonal antibodies and indirect competitive enzyme-linked immunosorbent assay kits for the detection of clenbuterol and salbutamol in the tissues and products of food-producing animals. Food Anal Methods 10:3623–3633
Li Y, Qi P, Ma X, Zhong JG (2014) Quick detection technique for clenbuterol hydrochloride by using surface plasmon resonance biosensor. Eur Food Res Technol 239:195–201
Hu JD, Wang S, Wang TT, Zhao YY, Li JW, Hu XR, Liang H, Zhu JH, Sun XH, Ma LZ, Jiang M (2015) Detection of clenbuterol hydrochloride residuals in pork liver using a customized surface plasmon resonance bioanalyzer. PLoS ONE 10:e0122005
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22064016), Natural Science Foundation of Xinjiang Uygur Autonomous Region (2019D01A69, 2019D01B36), Xinjiang Uygur Autonomous Region University Scientific Research Program Key Project (XJEDU2019I019), the Scientific Research and Development Project of Xinjiang Normal University (XJNUZX202003), the Doctoral Research and Innovation Program of Xinjiang Normal University (XJ107622007), Xinjiang Uygur Autonomous Region University Scientific Research Program Youth Project (XJEDU2018Y030).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All the authors declare that there are no conflicts of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peng, F., Li, B., Sun, S. et al. A novel fluorescence internal filtration immunoassay for the detection of clenbuterol. Eur Food Res Technol 248, 415–426 (2022). https://doi.org/10.1007/s00217-021-03886-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-021-03886-9