Abstract
In the present investigation, glucosinolate content was identified and quantified in 210 accessions of Korean leaf mustard (Brassica juncea var. integrifolia) by a liquid chromatography (LC) with electrospray ionization (ESI) coupled with a positive-mode ion trap tandem mass spectrometry (LC–ESI–MS/MS). Eleven individual GSLs including two new compounds were identified in the accessions using desulfo-glucosinolates (DS-GSLs) LC–MS library, and they were elucidated by interpreting the fragmentation data ([M + H-glucose]+) obtained. The newly identified GSLs contained aliphatic and hydroxyl functional group in their back bone were named as 1-ethyl-2-hydroxyethyl DS-GSLs (glucosisautricin) or 2-hydroxy-2-methylpropyl DS-GSLs (glucoconringiin) and 3,4,5-trimethoxybenzyl DS-GSLs, respectively. Among all tested accessions, the total DS-GSLs content was found to be varied between 5.3 and 23.2 mg/g dry weight (DW) with an average of 13.0 mg/g DW in the germplasm. Each individual DS-GSLs component was found in decreasing order of sinigrin (41.7 %) >glucoiberverin (21.7 %) >gluconasturtiin (12.6 %) >glucobarbarin (10.0 %) >glucoiberin (5.1 %) >glucocheirolin (3.6 %) >glucobrassicanapin (2.6 %) >gluconapin (2.1 %), and >glucobrassicin (0.6 %). Interestingly, sinigrin and gluconasturtiin were present in higher content, and progoitrin was not detected significantly in the germplasm of Korean leaf mustard. In particular, accession K046197 (purple) was found to show highest total DS-GSL content (23.2 mg/g DW).
Similar content being viewed by others
Introduction
Generally, plants produce a variety of toxic and repellent secondary metabolites to protect themselves against pathogens [1]. Among various substances involved in plant defense, glucosinolates (GSLs), major sulfate-containing secondary metabolites, were found in many agricultural crops such as cabbage, broccoli, radish, turnip, swede, kale, mustard, cauliflower, watercress, salad rocket, and rapeseed [27]. GSLs are responsible for diverse physiological effects such as inhibitors of microbial growth, attractants for particular insects, and as deterrents of different herbivores. Intact GSLs are nontoxic; however, upon tissue damage (e.g., by cutting or chewing), they come into contact with myrosinases and are hydrolyzed into unstable aglycones, which rearrange into a range of bioactive products and sometimes toxic compounds including isothiocyanates, thiocyanates, nitriles, oxazolidine-2-thiones, or epithioalkanes [2]. Although certain GSLs derivatives have anti-nutritional properties [25], it is now well established that methionine-derived isothiocyanates can offer substantial protection against cancer [18].
The different biological profiles of GSLs are the reason why these plant secondary metabolites attract the attention of several investigators [5, 15]. To date, more than 200 GSL structures have been described, although only some of these are commonly found within crop plants. In fact, most plants contain only a limited number of major GSLs (typically six or less) with a few others present in trace amounts [7, 12, 14]. All GSLs have a common core structure comprising a β-d-thioglucose group linked to a sulfonatedaldoxime moiety and a variable side chain derived from amino acids. Generally, they are grouped into aliphatic, aromatic, and indole GSLs depending on whether they are originated from aliphatic amino acids (methionine, alanine, valine, leucine and isoleucine), aromatic amino acids (tyrosine and phenylalanine), or tryptophan [7, 12]. The structural diversity of GSLs is due to chain elongations of amino acids before the formation of the glucosinolate core structure and secondary modifications of the GSLs side chain (e.g., thiol oxidation, hydroxylation, etc.) and/or the glucose moiety (esterification) [3]. In this regard, a high-performance liquid chromatography (HPLC) coupled to mass spectrum (MS) has generally been applied for GSL quantification.
Leaf mustard (Brassica juncea var. integrifolia; mustard green or red giant mustard) is a representative member of the Cruciferae family and is a major ingredient in Gat-Kimchi (leaf mustard Kimchi), which is known for its unique pungent and hot flavor. Kimchi can be classified according to the raw ingredients and processing methods used and the harvest season and location of the raw ingredients. 3-Isothiocyanate-1-propene (allylisothiocyanate; AITC), which is derived from sinigrin, has been identified as the major volatile compound contained in this dish. Depending on the processing conditions employed, the AITC content has been shown to decrease during fermentation, while the sinigrin content increases until the dish has ripened optimally [8, 24]. The antioxidant, cytotoxic, and anti-hypertensive effects of the dish were shown to correlate with the GSLs hydrolysis product content in Dolsan leaf mustard juice [33, 34].
Although a number of investigations on GSLs in leaf mustard have been reported in recent years, there is an inadequate qualitative and quantitative analysis of GSLs in the germplasms of Korean leaf mustard (Brassica juncea var. integrifolia). Thus, this study intended to analyze of GSLs from the germplasm of Korean leaf mustard using a high-performance liquid chromatography with electrospray ionization coupled with a positive-mode ion trap tandem mass spectrometry (LC–ESI–MS/MS).
Materials and methods
Materials
A total of 210 accessions of Korean leaf mustard (Brassica juncea var. integrifolia) included 29 green accessions and 181 purple accessions were compared with major vegetables in the Brassicaceae family, such as B. oleracea var. capitata (cabbage), B. rapa ssp. pekinensis (Chinese cabbage), Raphanus sativus L. (radish) and B. oleracea var. italic (broccoli). The samples were grown at the National Agro-biodiversity Center, National Academy of Agricultural Science (NAAS), Rural Development Administration (RDA), Republic of Korea in 2012. After harvesting, the leaves of each accession were ground finely with liquid nitrogen for the analyses of GSLs.
Extraction and desulfation of glucosinolates
Each powdered sample (0.1 g) was frozen and lyophilized, and then they are dissolved by boiling aqueous MeOH (70 %, 1.5 mL) in a microcentrifuge tube (2.0 mL). This process rendered the hydrolytic enzyme myrosinase which responsible for the hydrolysis of glucosinolates. Then each samples was extracted for 5 min in a water bath at 70 °C and then separated by centrifugation (12,000 rpm, 10 min, 4 °C) [7]. This process was repeated for two times to allow the complete extraction of the residue and the supernatants obtained from each extraction were combined. The combined extract was loaded onto a mini-column filled with DEAE-Sephadex A-25, 75 μL of aryl sulfatase was injected onto the column, and the GSLs present in the extract were desulfated at room temperature for 16–18 h. Desulfo-glucosinolates (DS-GSLs) were then eluted into a microcentrifuge tube (2.0 mL) using three aliquots (0.5 mL each) of distilled water. The eluted extract was concentrated by nitrogen gas and dissolved in distilled water (200 μL) and stored at −20 °C before undergoing HPLC analysis.
Identification and quantification of DS-GSLs by LC–ESI–MS/MS and HPLC
For the qualitative and quantitative analysis of DS-GSLs, HPLC–MS (Finnigan LCQ Deca XP MAX ion trap mass spectrometer, Thermo Scientific, USA) with electrospray ionization detection was carried out using an Inertsil ODS-3 reversed phase column (2.1 × 150 mm I.D., 5 μm; GL Sciences, Japan), at a flow rate of 0.2 mL/min and a detection wavelength of 227 nm with a column temperature of 35 °C. The mobile phases used were 1 % formic acid in water (phase A) and 0.1 % formic acid in 20 % acetonitrile (phase B), and the pretreated sample was analyzed using the following elution conditions: gradient elution from 10 to 90 % phase B over 23 min, isocratic elution at 90 % phase B for 9 min, gradient elution from 90 to 10 % phase B over 3 min, and finally re-equilibration of the column with 10 % phase B for 5 min. MS/MS analysis was conducted in a positive ionization mode using electrospray ionization (ESI) source with the following MS parameters: cone voltage of 3.5 kV, capillary temperature of 250 °C, and desolvation N2 gas flow rate of 300 L/h. The molecular weight range observed was 50–800 m/z in a full-scan mode. The DS-GSL content of each sample was calculated using the HPLC peak area and the relative response factor [7] of individual ingredients against sinigrin, which was used as an external standard [4, 30]. In addition, to efficiently check individual component, a LC–MS library of 112 naturally occurring DS-GSLs was constructed using the data taken from the literature.
Results and discussion
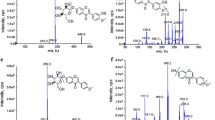
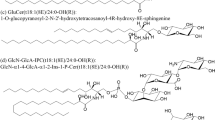
In this study, LC–MS DS-GSL library of Brassica juncea var. integrifolia revealed a total of 11 individual components including two new compounds. This was compared with the results of Carlson et al. [9] who identified four components (sinigrin, gluconapin, glucobrassicin, and glucosnasturtiin) and Cole [11] who identified seven components (glucoiberin, sinigrin, gluconapin, glucoiberverin, glucobrassicanapin, glucobrassicin, and gluconasturtiin) from the leaves of B. juncea. The chemical structure of the individual DS-GSLs was determined by interpreting fragmentation patterns observed by ion trap MS/MS with ESI detection. Though molecular ions were not observed, the fragmentation patterns of [M + H-glucose]+ ions of the DS-GSLs were used for the analysis [17, 19]. The result revealed that one of the unknown DS-GSLs was found to have a molecular weight of 311 (m/z 311.65, [M + H]+) and was potentially assigned as either 1-ethyl-2-hydroxyethyl DS-GSLs (glucosisautricin) or 2-hydroxy-2-methylpropyl DS-GSLs (glucoconringiin), whereas the other unknown DS-GSLs was presumed as hydroxy-3,4,5-trimethoxybenzyl DS-GSLs with a molecular weight of 439 (m/z 439.72, [M + H]+) (Fig. 1) [6].
Mass spectra and interpretation of unknown DS-GSLs in Korean leaf mustard by LC–ESI–MS/MS and the DS-GSLs LC–MS library. a 1-Ethyl-2-hydroxyethyl DS-GSLs: glucosisautricin or 2-hydroxy-2-methylpropyl DS-GSLs: glucoconringin (assigned unknown compound) [6]; b hydroxy-3,4,5-trimethoxybenzoyl DS-GSLs (presumed unknown compound) [7]
This present study showed that sinigrin, glucobarbarin and gluconasturtiin were the major components which were confirmed by their molecular ion and the common fragmentation pattern of the loss of glucose (Fig. 2).
Of the 210 accessions of Korean leaf mustard (29 green accessions and 181 purple accessions), content distribution of individual DS-GSLs appeared between 5.3 and 23.2 mg/g DW with an average DS-GSLs content of 13.0 mg/g DW. Among them, 128 accessions (61.0 %) showed the most population DS-GSL proportion of 10–15 mg/g DW (Fig. 3).
Each individual DS-GSL component was estimated to occur the higher content than the earlier reports [9, 11] with the following components which were detected in decreasing order of their content as sinigrin (41.7 %) >glucoiberverin (21.7 %) >gluconasturtiin (12.6 %) >glucobarbarin (10.0 %) >glucoiberin (5.1 %) >glucocheirolin (3.6 %) >glucobrassicanapin (2.6 %) >gluconapin (2.1 %), and >glucobrassicin (0.6 %). The sinigrin was confirmed as the predominant GSL among others in B. juncea germplasm [13, 28]. The accession K046145 exhibited the highest sinigrin content (9.38 mg/g DW, purple) with a total DS-GSL content (16.4 mg/g DW). However, accession K046197 (purple) showed the highest content of gluconapin (4.11 mg/g DW) and also highest total DS-GSL content (23.2 mg/g DW) than the other accessions studied, and gluconapin was found in trace quantities in most accessions (Table 1).
In particular, as compared with the four commonly consumed vegetables belonging to the Brassicaceae family tested, the total DS-GSL content of B. juncea was the highest with approximately twice as much DS-GSLs in B. oleracea var. capitata (cabbage) or B. campestris ssp. pekinensis (Chinese cabbage). These results were reproved by earlier studies which have previously been reported as 10.9 μmol (approx. 4.5 mg/g DW) for B. oleracea var. capitata [20] and 4.5–31.6 μmol (approx. 1.3–8.8 mg/g DW) for B. campestris ssp. [21]. Progoitrin, the precursor of goitrin (5-vinyloxazolidine-2-thione) as the thyroid toxin, was not detected in the Korean leaf mustard germplasm. Sinigrin and gluconasturtiin were higher than other vegetables in the Brassicaceae family (Table 2). The degradation product of sinigrin, namely allylisothiocyanate (AITC), has a unique pungent and spicy taste that is characteristics of the leaf mustard [8] and is known to effectively inhibit carcinogenesis by inducing cell cycle arrest and apoptosis in prostate, ovarian and liver cancers (Musk et al. [26]; [22, 32]). In addition, the degradation product of gluconasturtiin, phenethylisothiocyanate (PEITC), is a potential carcinogenesis inhibitor in lung, liver, prostate, and ovarian cancers (Smith et al. [30]; [10, 16, 29, 32]) and is certified as being among the 40 most important anticancer drugs by the National Cancer Institute (NCI) in the USA [23]. Nugrahedi et al. [28] reported the loss of GSL sinigrin during fermentation Indian mustard leaves in the production of sayur asin. This result was considered that sinigrin was converted to potentially AITC during fermentation process. Thus, the Korean leaf mustard as the main dish in which it is used, such as Kimchi, was considered as cancer preventive foodstuffs for higher sinigrin and gluconasturtiin contents. Our results are therefore consistent with the previous reports as dry weight DS-GSL content but slightly higher as fresh weight content. This difference may be accounted by differences in the varieties used as well as number and peak areas of components observed by HPLC analysis.
Conclusion
In conclusion, individual DS-GSLs were evaluated using a liquid chromatography coupled with ion trap tandem mass spectrometry of electrospray ionization (ESI) and positive-mode types (LC–ESI–MS/MS) in 210 accessions of Korean leaf mustard (Brassica juncea var. integrifolia), and a total of 11 components including two new compounds namely 1-ethyl-2-hydroxyethyl DS-GSLs (glucosisautricin) or 2-hydroxy-2-methylpropyl DS-GSLs (glucoconringiin) and 3,4,5-trimethoxybenzyl DS-GSLs were identified. Among all tested accessions, the total DS-GSL content was found to be varied between 5.3 and 23.2 mg/g DW with an average of 13.0 mg/g dry weight (DW) in the germplasm. Interestingly, sinigrin (41.7 %) and gluconasturtiin (12.6 %) were present in higher content, and progoitrin was not detected significantly in the germplasm of Korean leaf mustard than the other vegetables. In particular, accession K046197 (purple) was found to show the highest, total DS-GSL content (23.2 mg/g DW).
References
Ananthakrishnan TN (2001) Phytochemicals as insect behaviour modifiers. In: Koul O, Dhaliwal GS (eds) Phytochemical biopesticides. Harwood Academic Publishers, Amsterdam, p 5
Agerbirk N, Vos MD, Kim JH, Jander G (2009) Indoleglucosinolate breakdown and its biological effects. Phytochem Rev 8:101–120
Agerbirk N, Olsen CE (2012) Glucosinolate structures in evolution. Phytochemistry 77:16–45
Baenas N, Moreno DA, Garcia-Viguera C (2012) Selecting sprouts of Brassicaceae for optimum phytochemical composition. J Agric Food Chem 60:11409–11420
Brown J, Morra MJ (1995) Glucosinolate-containing plant tissues as bioherbicides. J Agric Food Chem 43:3070–3074
Brown J, Morra MJ (2005) Glucosinolate-containing seed meal as a soil amendment to control plant pests 2000–2002. University of Idaho Moscow, pp 73–75
Clarke DB (2010) Glucosinolates, structures and analysis in food. Anal Methods 2:310–325
Chun SS, Choi OJ, Cho YS, Park SK, Park JR (1995) Changes in pungent components of Dolsan leaf mustard Kimchi during fermentation. J Korean Soc Food Sci Nutr 24:54–59
Carlson DG, Daxenbichler ME, Vanetten CH, Kwolekand WF, Williams PH (1987) Glucosinolates in crucifer vegetables: broccoli, brussels sprouts, cauliflower, collards, kale, mustard green, and kohlrabi. J Am Soc Hortic Sci 112:173–178
Chen YR, Han J, Kori R, Kong ANT, Tan TH (2002) Phenylehtylisothiocyanate induce apoptotic signaling via suppressing phosphatase activity against c-Jun N-terminal kinase. J Biol Chem 277:39334–39342
Cole RA (1997) The relative importance of glucosinolates and amino acids to the development of two aphid pests Brevicorynebrassicae and Myzuspersicae on wild and cultivated brassica species. Entomol Exp Appl 85:121–133
Fahey JW, Zalcmann AT, Talalay P (2001) The chemical diversity and distribution of glucosinolates and isothiocyanates among plnats. Phytochemistry 56:5–51
Font R, Del Rio M, Fernandez-Martinez JM, De Haro-Bailon A (2004) Use of near-infrared spectroscopy for screening the individual and total glucosinolate contents in Indian mustard seed (Brassica juncea L. Czern. & Coss.). J Agric Food Chem 52:3563–3569
Halkier BA, Gershenzon J (2012) Biology and biochemistry of glucosinolates. Annu Rev Plant Biol 57:303–333
Hanley AB, Parsley KR (1990) Identification of 1-methoxyindolyl-3-methyl isothiocyanate as an indole glucosinolate breakdown product. Phytochemistry 29:769–771
Hecht SS (1999) Chemoprevention of cancer by isothiocyanates, modifiers of carcinogen metabolism. J Nutr 129:768–774
Ishida M, Chiba I, Okuyama Y, Takahata Y, Kaizuma N (1997) Separation and identification of desulfoglucosinolates in Japanese rapeseed by LC/APCI-MS. Jpn Agric Res Q 31:73–80
Keum YS, Jeong WS, Kong ANT (2004) Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutat Res 555:191–202
Kim SJ, Kawaharada C, Jin S, Hashimoto M, Ishii G, Yamaguchi H (2007) Structural elucidation of 4-(cystein-S-yl)butylglucosinolate from the leaves of Eruca sativa. Biosci Biotechnol Biochem 71:114–121
Kushad MM, Brown AF, Kurilich AC, Juvik JA, Klein BP, Wallig MA, Jeffery EH (1999) Variation of glucosinolates in vegetable crops of Brassica oleracea. J Agric Food Chem 47:1541–1548
Kim JK, Chu SM, Kim SJ, Lee DJ, Lee SY, Lim SH, Ha SH, Kweon SJ, Cho HS (2010) Variation of glucosinolates in vegetable crops of Brassica rapa L. ssp. pekinensis. Food Chem 119:423–428
Kassie F, Knasmuller S (2000) Genotoxic effects of allylisothiocyanate (AITC) and phenethylisothiocyanate (PEITC). Chem Biol Interact 127:163–180
Kelloff GJ, Crowell JA, Steele VE, Lubet RA, Malone WA, Boone CW, Kopelovich L, Hawk ET, Lieberman R, Lawrence JA, Ali I, Viner JL, Sigman CC (2000) Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr 130:467–471
Lim HS (2002) The study for contents of sinigrin in Dolsan leaf mustard Kimchi during fermentation periods. Korean J Life Sci 12:523–527
Mithen R (2001) Glucosinolates and their degradation products. Adv Bot Res 35:213–262
Musk SRR, Smith TK, Johnson IT (1995) On the cytotoxicity and genotoxicity of allyl and phenethyl isothiocyanates and their parent glucosinolates singrin and gluconasturtiin. Mutat Res 348:19–23
Nugon-Baudon L, Rabot S (1994) Glucosinolates and glucosinolate derivatives: implications for protection against chemical carcinogenesis. Nutr Res Rev 7:205–231
Nugrahedi PY, Widianarko B, Dekker M, Verkerk R, Oliviero T (2015) Retention of glucosinolates during fermentation of Brassica juncea: a case study on production of sayur asin. Eur Food Res Technol 240:559–565
Satyan KS, Swamy N, Dizon DS, Singh R, Granai CO, Brard L (2006) Phenethylisothiocyanate (PEITC) inhibits growth of ovarian cancer cells by inducing apoptosis: role of caspase and MAPK activation. Gynecol Oncol 103:261–270
Smith TJ, Guo Z, Guengerich FP, Yang CS (1996) Metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) by human cytochrome P450 1A2 and its inhibition by phenethyl isothiocyanate. Carcinog 17:809–813
Vallejo F, Tomas-Barberan FA, Garcia-Viguera C (2002) Glucosinolates and vitamin C content in edible parts of broccoli florets after domestic cooking. Eur Food Res Technol 215:310–316
Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, Trump DL, Singh SV (2003) Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis 24:891–897
Yoo EJ, Choi MR, Lim HS (2004) The relationship between ACE inhibitory activity and degradations of sulfur containing materials in Dolsan leaf mustard juice. Biotechnol Bioprocess Eng 9:400–404
Yoo EJ, Lim HS, Park KO, Choi MR (2005) Cytotoxic, antioxidative and ACE inhibiting activities of Dolsan leaf mustard juice (DLMJ) treated with lactic acid bacteria. Biotechnol Bioprocess Eng 10:60–66
Acknowledgments
This study was carried out with the support of “Research Program for Agricultural Science and Technology Development (Project No. PJ010052),” National Academy of Agricultural Science, Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kim, H.W., Ko, H.C., Baek, H.J. et al. Identification and quantification of glucosinolates in Korean leaf mustard germplasm (Brassica juncea var. integrifolia) by liquid chromatography–electrospray ionization/tandem mass spectrometry. Eur Food Res Technol 242, 1479–1484 (2016). https://doi.org/10.1007/s00217-016-2648-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-016-2648-6