Abstract
Cu isotope fractionation was investigated in the human neuroblastoma SH-SY5Y cell line, in a proliferating/tumor phase (undifferentiated cells), and in a differentiated state (neuron-like cells), induced using retinoic acid (RA). The SH-SY5Y cell line displays genetic aberrations due to its cancerous origin, but differentiation drives the cell line towards phenotypes suitable for the research of neurological diseases (e.g., Alzheimer’s disease or Parkinson’s disease). Cellular Cu distribution was first explored by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) imaging and, subsequently, Cu isotopic analysis was performed at cellular and sub-cellular levels via multi-collector inductively coupled plasma-mass spectrometry (MC-ICP-MS). The SH-SY5Y cells showed a re-distribution of intracellular Cu upon RA differentiation. Both undifferentiated and differentiated cells became systematically enriched in the light 63Cu isotope with increasing intracellular Cu content. Differentiated neuron-like SH-SY5Y cells showed a heavier Cu isotopic composition (+ 0.3‰) than did the undifferentiated proliferating cells when exposed to Cu for 24 h. However, after a longer exposure time (72 h), no difference was observed between both cellular phenotypes. Mitochondrial fractions were enriched in the light 63Cu isotope, compared to whole cells, for both undifferentiated and differentiated cells (no significant difference). The Cu isotopic composition of the remaining cell lysates was heavier than that of the whole cells and + 0.2‰ heavier in the differentiated cells than in the undifferentiated cells. These results indicate that neuronal differentiation affects the Cu isotope fractionation accompanying Cu uptake in the cells, but this effect does not seem to be associated with the mitochondrial Cu pathway. Cu isotope fractionation can be an interesting tool for studying Cu metabolism at a (sub)-cellular level in functional neurons.

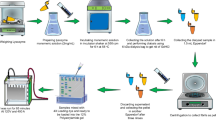
Graphical abstract





Similar content being viewed by others
References
Xicoy H, Wieringa B, Martens GJM. The SH-SY5Y cell line in Parkinson’s disease research: a systematic review. Mol Neurodegener. 2017;12:10.
Cheung YT, Lau WK, Yu MS, Lai CS, Yeung SC, So KF, et al. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology. 2009;30:127–35.
Martins Lopes F, Schröder R, Conte da Frota ML Jr, Zanotto-Filho A, Müller CB, Simões Pires A, et al. Comparison between proliferative and neuron-like SH-SY5Y cells as an in vitro model for Parkinson disease studies. Brain Res. 2010;1337:85–94.
Kovalevich J, Langford D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol Biol. 2013;1078:9–21.
Encinas M, Iglesias M, Liu Y, Wang H, Muhaisen A, Ceña V, et al. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem. 2000;75:991–1003.
Miloso M, Villa D, Crimi M, Galbiati S, Donzelli E, Nicolini G, et al. Retinoic acid-induced neuritogenesis of human neuroblastoma SH-SY5Y cells is ERK independent and PKC dependent. J Neurosci Res. 2004;75:241–52.
Pahlman S, Ruusala AI, Abrahamsson L, Mattsson ME, Esscher T. Retinoic acid-induced differentiation of cultured human neuroblastoma cells: a comparison with phorbolester-induced differentiation. Cell Differ. 1984;14:135–44.
Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. J Clin Oncol. 2009;27:1007–13.
Bohlken A, Cheung BB, Bell JL, Koach J, Smith S, Sekyere E, et al. ATP7A is a novel target of retinoic acid receptor b2 in neuroblastoma cells. Brit J Cancer. 2009;100:96–105.
Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–80.
López-Carballo G, Moreno L, Masia S, Perez P, Barettino D. Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway by retinoic acid is required for neuronal differentiation of SH-SY5Y human neuroblastoma cells. J Biol Chem. 2002;277:25297–304.
Hatori Y, Yan Y, Schmidt K, Furukawa E, Hasan NM, Yang N, et al. Neuronal differentiation is associated with a redox-regulated increase of copper flow to the secretory pathway. Nat Commun. 2016;7:10640.
Mega MS. The cholinergic deficit in Alzheimer’s disease: impact on cognition, behaviour and function. Int J Neuropsychopharmacol. 2003;3:S3–S12.
Lutsenko S, Bhattacharjee A, Hubbard AL. Copper handling machinery of the brain. Metallomics. 2010;2:596–608.
Liu T, Bohlken A, Kuljaca S, Lee M, Nguyen T, Smith S, et al. The retinoid anticancer signal: mechanisms of target gene regulation. Br J Cancer. 2005;93:310–8.
Xun Z, Lee DY, Lim J, Canaria CA, Barnebey A, Yanonne SM, et al. Retinoic acid-induced differentiation increases the rate of oxygen consumption and enhances the spare respiratory capacity of mitochondria in SH-SY5Y cells. Mech Ageing Dev. 2012;133:176–85.
Truckenmiller ME, Vawter MP, Cheadle C, Coggiano M, Donovan DM, Willian J, et al. Gene expression profile in early stage of retinoic acid-induced differentiation of human SH-SY5Y neuroblastoma cells. Restor Neurol Neurosci. 2001;18:67–80.
Costas-Rodríguez M, Delanghe J, Vanhaecke F. High-precision isotopic analysis of essential mineral elements in biomedicine: natural isotope ratio variations as potential diagnostic and/or prognostic markers. TrAC Trends Anal Chem. 2016;76:182–93.
Lauwens S, Costas-Rodríguez M, Van Vlierberghe H, Vanhaecke F. Cu isotopic signature in blood serum of liver transplant patients: a follow-up study. Sci Rep. 2016;6:30683.
Balter V, da Costa AN, Bondanese VP, Jaouen K, Lamboux A, Sangrajrang S, et al. Natural variations of copper and sulfur stable isotopes in blood of hepatocellular carcinoma patients. Proc Natl Acad Sci U S A. 2015;112:982–5.
Télouk P, Puisieux A, Fujii T, Balter V, Bondanese V, Morel AP, et al. Copper isotope effect in serum of cancer patients. A pilot study. Metallomics. 2015;7:299–308.
Costas-Rodríguez M, Anoshkina Y, Lauwens S, Van Vlierberghe H, Delanghe J, Vanhaecke F. Isotopic analysis of Cu in blood serum by multi-collector ICP-mass spectrometry: a new approach for the diagnosis and prognosis of liver cirrhosis? Metallomics. 2015;7:491–8.
Lobo L, Costas-Rodríguez M, de Vicente JC, Pereiro VF, Sanz-Medel A. Elemental and isotopic analysis of oral squamous cell carcinoma tissues using sector-field and multi-collector ICP-mass spectrometry. Talanta. 2017;165:92–7.
Sauzéat L, Bernard E, Perret-Liaudet A, Quadrio I, Vighetto A, Krolak-Salmon P, et al. Isotopic evidence for disrupted copper metabolism in amyotrophic lateral sclerosis. iScience. 2018;31:264–71.
Miller KA, Keenan CM, Martin GR, Jirik FR, Sharkey KA, Wieser ME. The expression levels of cellular prion protein affect copper isotopic shifts in the organs of mice. J Anal At Spectrom. 2016;31:2015–22.
Paredes E, Avazeri E, Malard V, Vidaud C, Reiller PE, Ortega R, et al. Impact of uranium uptake on isotopic fractionation and endogenous element homeostasis in human neuron-like cells. Sci Rep. 2018;8:17163.
Flórez MR, Costas-Rodríguez M, Grootaert C, Van Camp J, Vanhaecke F. Cu isotope fractionation response to oxidative stress in a hepatic cell line studied using multi-collector ICP-mass spectrometry. Anal Bioanal Chem. 2018;410:2385–94.
Cadiou JL, Pichat S, Bondanese VP, Soulard A, Fujii T, Albarède F, et al. Copper transporters are responsible for copper isotopic fractionation in eukaryotic cells. Sci Rep. 2017;7:44533.
Paredes E, Avazeri E, Malard V, Vidaud C, Reiller PE, Ortega R, et al. Evidence of isotopic fractionation of natural uranium in cultured human cells. Proc Natl Acad Sci U S A. 2016;113:14007–12.
Bondanese VP, Lamboux A, Simon M, Lafont JE, Albalat E, Pichat S, et al. Hypoxia induces copper stable isotope fractionation in hepatocellular carcinoma, in a HIF-independent manner. Metallomics. 2016;8:1177–84.
Tashtoush BM, Jacobson EL, Jacobson MK. UVA is the major contributor to the photodegradation of tretinoin and isotretinoin: implications for development of improved pharmaceutical formulations. Int J Pharm. 2008;352:123–8.
Lauwens S, Costas-Rodríguez M, Vanhaecke F. Ultra-trace Cu isotope ratio measurements via multi-collector ICP-mass spectrometry using Ga as internal standard: an approach applicable to micro-samples. Anal Chim Acta. 2018;1028:69–79.
Van Malderen SJM, van Elteren JT, Vanhaecke F. Development of a fast laser ablation-inductively coupled plasma-mass spectrometry cell for sub-μm scanning of layered materials. J Anal At Spectrom. 2015;30:119–25.
Van Malderen SJM, Van Acker T, Vanhaecke F. Fast, but not furious: development & analytical advantages of the cobalt ultrafast ablation cell. Laser ablation, 14th European workshop, Pau (France), 2018.
Van Acker T, Van Malderen SJM, Buckle T, Vanhaecke F. High-speed sub-micrometer laser-ablation inductively coupled plasma-mass spectrometry imaging for a DNA-binding 103Rh intercalator in single cells using the ARIS. Application note: ANALYTE G2–003, Teledyne CETAC Technologies Inc., 2017.
Arciello M, Rotilio G, Rossi L. Copper-dependent toxicity in SH-SY5Y neuroblastoma cells involves mitochondrial damage. Biochem Biophys Res Commun. 2005;327:454–9.
Constantinescu R, Constantinescu AT, Reichmann H, Janetzky B. Neuronal differentiation and long-term culture of the human neuroblastoma line SH-SY5Y. J Neural Transm. 2007;72:17–28.
Dodani SC, Domaille DW, Nam CI, Miller EW, Finney LA, Vogt S, Chang CJ. Calcium-dependent copper redistributions in neuronal cells revealed by a fluorescent copper sensor and X-ray fluorescence microscopy. Proc Natl Acad Sci U S A 2011¸108:5980–5985.
Korecka JA, van Kesteren RE, Blaas E, Spitzer SO, Kamstra JH, Smit AB, et al. Phenotypic characterization of retinoic acid differentiated SH-SY5Y cells by transcriptional profiling. PLoS One. 2013;8:e63862.
Urso E, Rizzello A, Acierno R, Lionetto MG, Salvato B, Storelli C, et al. Fluorimetric analysis of copper transport mechanisms in the B104 neuroblastoma cell model: a contribution from cellular prion protein to copper supplying. J Membr Biol. 2010;233:13–21.
Lindahl PA, Moore MJ. Labile low-molecular-mass metal complexes in mitochondria: trials and tribulations of a burgeoning field. Biochemistry. 2016;55:4140–53.
Cobine PA, Pierrel F, Bestwick ML, Winge DR. Mitochondrial matrix copper complex used in metalation of cytochrome oxidase and superoxide dismutase. J Biol Chem. 2006;281:36552–99.
Leary SC, Winge DR, Cobine PA. “Pulling the plug” on cellular copper: the role of mitochondria in copper export. Biochim Biophys Acta, Mol Cell Res. 2009;1793:146–53.
Acknowledgements
Marta Costas-Rodríguez thanks FWO-Vlaanderen for her postdoctoral grant. Legna Colina-Vegas acknowledges the São Paulo Research Foundation (Fapesp grant #2017/23254-9). The authors would like to acknowledge Teledyne CETAC Technologies Inc. for logistic support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Published in the topical collection Young Investigators in (Bio-)Analytical Chemistry with guest editors Erin Baker, Kerstin Leopold, Francesco Ricci, and Wei Wang.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Costas-Rodríguez, M., Colina-Vegas, L., Solovyev, N. et al. Cellular and sub-cellular Cu isotope fractionation in the human neuroblastoma SH-SY5Y cell line: proliferating versus neuron-like cells. Anal Bioanal Chem 411, 4963–4971 (2019). https://doi.org/10.1007/s00216-019-01871-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01871-6