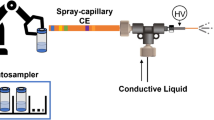
Abstract
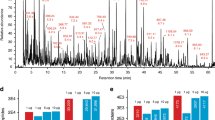
Mass spectrometry (MS)–based analysis of complex biological samples is essential for biomedical research and clinical diagnostics. The separation prior to MS plays a key role in the overall analysis, with separations having larger peak capacities often leading to more identified species and improved confidence in those identifications. High-resolution ion mobility (IM) separations enabled by Structures for Lossless Ion Manipulation (SLIM) can provide extremely rapid, high-resolution separations and are well suited as a second dimension of separation following nanoscale liquid chromatography (nanoLC). However, existing sample handling approaches for offline coupling of separation modes require microliter-fraction volumes and are thus not well suited for analysis of trace biological samples. We have developed a novel nanowell-mediated fractionation system that enables nanoLC-separated samples to be efficiently preconcentrated and directly infused at nanoelectrospray flow rates for downstream analysis. When coupled with SLIM IM-MS, the platform enables rapid and high-peak-capacity multidimensional separations of small biological samples. In this study, peptides eluting from a 100 nL/min nanoLC separation were fractionated into ~ 60 nanowells on a microfluidic glass chip using an in-house–developed robotic system. The dried samples on the chip were individually reconstituted and ionized by nanoelectrospray for SLIM IM-MS analysis. Using model peptides for characterization of the nanowell platform, we found that at least 80% of the peptide components of the fractionated samples were recovered from the nanowells, providing up to ~tenfold preconcentration for SLIM IM-MS analysis. The combined LC-SLIM IM separation peak capacities exceeded 3600 with a measurement throughput that is similar to current one-dimensional (1D) LC-MS proteomic analyses.

A nanowell-mediated multidimensional separation platform that combines nanoLC with SLIM IM-MS enables rapid, high-peak-capacity proteomic analyses.





Similar content being viewed by others
References
Ong S-E, Mann M. Mass spectrometry–based proteomics turns quantitative. Nat Chem Biol. 2005;1(5):252–62.
Bantscheff M, Schirle M, Sweetman G, Rick J, Kuster B. Quantitative mass spectrometry in proteomics: a critical review. Anal Bioanal Chem. 2007;389(4):1017–31.
Angel TE, Aryal UK, Hengel SM, Baker ES, Kelly RT, Robinson EW, et al. Mass spectrometry-based proteomics: existing capabilities and future directions. Chem Soc Rev. 2012;41(10):3912–28.
Zhu Y, Zhao R, Piehowski PD, Moore RJ, Lim S, Orphan VJ, et al. Subnanogram proteomics: impact of LC column selection, MS instrumentation and data analysis strategy on proteome coverage for trace samples. Int J Mass Spectrom. 2017;427:4–10.
Boschetti E, Righetti PG. Low-abundance proteome discovery: state of the art and protocols. Oxford: Elsevier; 2013.
Dakna M, Harris K, Kalousis A, Carpentier S, Kolch W, Schanstra JP, et al. Addressing the challenge of defining valid proteomic biomarkers and classifiers. BMC Bioinformatics. 2010;11(1):594.
Drucker E, Krapfenbauer K. Pitfalls and limitations in translation from biomarker discovery to clinical utility in predictive and personalised medicine. EPMA J. 2013;4(1):7.
Choi YS. Reaching for the deep proteome: recent nano liquid chromatography coupled with tandem mass spectrometry-based studies on the deep proteome. Arch Pharm Res. 2012;35(11):1861–70.
Karas M, Bahr U, Dülcks T. Nano-electrospray ionization mass spectrometry: addressing analytical problems beyond routine. Fresenius J Anal Chem. 2000;366(6–7):669–76.
Köcher T, Swart R, Mechtler K. Ultra-high-pressure RPLC hyphenated to an LTQ-Orbitrap Velos reveals a linear relation between peak capacity and number of identified peptides. Anal Chem. 2011;83(7):2699–704.
Zubarev RA. The challenge of the proteome dynamic range and its implications for in-depth proteomics. Proteomics. 2013;13(5):723–6.
Furey A, Moriarty M, Bane V, Kinsella B, Lehane M. Ion suppression; a critical review on causes, evaluation, prevention and applications. Talanta. 2013;115:104–22.
Shen Y, Zhang R, Moore RJ, Kim J, Metz TO, Hixson KK, et al. Automated 20 kpsi RPLC-MS and MS/MS with chromatographic peak capacities of 1000−1500 and capabilities in proteomics and metabolomics. Anal Chem. 2005;77(10):3090–100.
Giddings JC. Concepts and comparisons in multidimensional separation. J Sep Sci. 1987;10(5):319–23.
Causon TJ, Hann S. Theoretical evaluation of peak capacity improvements by use of liquid chromatography combined with drift tube ion mobility-mass spectrometry. J Chromatogr A. 2015;1416:47–56.
Zhu M-Z, Li N, Wang Y-T, Liu N, Guo M-Q, Sun B-Q, et al. Acid/salt/pH gradient improved resolution and sensitivity in proteomics study using 2D SCX-RP LC–MS. J Proteome Res. 2017;16(9):3470–5.
Wang Y, Yang F, Gritsenko MA, Wang Y, Clauss T, Liu T, et al. Reversed-phase chromatography with multiple fraction concatenation strategy for proteome profiling of human MCF10A cells. Proteomics. 2011;11(10):2019–26.
Dou M, Zhu Y, Liyu A, Liang Y, Chen J, Piehowski P, et al. Nanowell-mediated two-dimensional liquid chromatography enables deep proteome profiling of <1000 mammalian cells. Chem Sci. 2018;9(34):6944–51.
Sommella E, Cacciola F, Donato P, Dugo P, Campiglia P, Mondello L. Development of an online capillary comprehensive 2D-LC system for the analysis of proteome samples. J Sep Sci. 2012;35(4):530–3.
Gilar M, Olivova P, Daly AE, Gebler JC. Orthogonality of separation in two-dimensional liquid chromatography. Anal Chem. 2005;77(19):6426–34.
Evans CR, Jorgenson JW. Multidimensional LC-LC and LC-CE for high-resolution separations of biological molecules. Anal Bioanal Chem. 2004;378(8):1952–61.
Chambers AG, Mellors JS, Henley WH, Ramsey JM. Monolithic integration of two-dimensional liquid chromatography−capillary electrophoresis and electrospray ionization on a microfluidic device. Anal Chem. 2011;83(3):842–9.
Mellors JS, Black WA, Chambers AG, Starkey JA, Lacher NA, Ramsey JM. Hybrid capillary/microfluidic system for comprehensive online liquid chromatography-capillary electrophoresis-electrospray ionization-mass spectrometry. Anal Chem. 2013;85(8):4100–6.
Baker ES, Livesay EA, Orton DJ, Moore RJ, Danielson Iii WF, Prior DC, et al. An LC-IMS-MS platform providing increased dynamic range for high-throughput proteomic studies. J Proteome Res. 2010;9(2):997–1006.
Webb IK, Garimella SV, Tolmachev AV, Chen TC, Zhang X, Norheim RV, et al. Experimental evaluation and optimization of structures for lossless ion manipulations for ion mobility spectrometry with time-of-flight mass spectrometry. Anal Chem. 2014;86(18):9169–76.
Webb IK, Garimella SV, Tolmachev AV, Chen TC, Zhang X, Cox JT, et al. Mobility-resolved ion selection in uniform drift field ion mobility spectrometry/mass spectrometry: dynamic switching in structures for lossless ion manipulations. Anal Chem. 2014;86(19):9632–7.
Tolmachev AV, Webb IK, Ibrahim YM, Garimella SV, Zhang X, Anderson GA, et al. Characterization of ion dynamics in structures for lossless ion manipulations. Anal Chem. 2014;86(18):9162–8.
Garimella SV, Ibrahim YM, Webb IK, Tolmachev AV, Zhang X, Prost SA, et al. Simulation of electric potentials and ion motion in planar electrode structures for lossless ion manipulations (SLIM). J Am Soc Mass Spectrom. 2014;25(11):1890–6.
Zhang XY, Garimella SVB, Prost SA, Webb IK, Chen TC, Tang KQ, et al. Ion trapping, storage, and ejection in structures for lossless ion manipulations. Anal Chem. 2015;87(12):6010–6.
Hamid AM, Ibrahim YM, Garimella SV, Webb IK, Deng L, Chen T-C, et al. Characterization of traveling wave ion mobility separations in structures for lossless ion manipulations. Anal Chem. 2015;87(22):11301–8.
Chen TC, Ibrahim YM, Webb IK, Garimella SV, Zhang X, Hamid AM, et al. Mobility-selected ion trapping and enrichment using structures for lossless ion manipulations. Anal Chem. 2016;88(3):1728–33.
Ibrahim YM, Hamid AM, Cox JT, Garimella SVB, Smith RD. Ion elevators and escalators in multilevel structures for lossless ion manipulations. Anal Chem. 2017;89(3):1972–7.
Deng L, Garimella SVB, Hamid AM, Webb IK, Attah IK, Norheim RV, et al. Compression ratio ion mobility programming (CRIMP) accumulation and compression of billions of ions for ion mobility-mass spectrometry using traveling waves in structures for lossless ion manipulations (SLIM). Anal Chem. 2017;89(12):6432–9.
Deng L, Webb IK, Garimella SVB, Hamid AM, Zheng X, Norheim RV, et al. Serpentine ultralong path with extended routing (SUPER) high resolution traveling wave ion mobility-MS using structures for lossless ion manipulations. Anal Chem. 2017;89(8):4628–34.
Zhu Y, Piehowski PD, Zhao R, Chen J, Shen Y, Moore RJ, et al. Nanodroplet processing platform for deep and quantitative proteome profiling of 10–100 mammalian cells. Nat Commun. 2018;9(1):882.
Zhu Y, Dou M, Piehowski PD, Liang Y, Wang F, Chu RK, et al. Spatially resolved proteome mapping of laser capture microdissected tissue with automated sample transfer to nanodroplets. Mol Cell Proteomics. 2018;17(9):1864–74.
Zhu Y, Clair G, Chrisler W, Shen Y, Zhao R, Shukla A, et al. Proteomic analysis of single mammalian cells enabled by microfluidic nanodroplet sample preparation and ultrasensitive nanoLC-MS. Angew Chem. 2018;130(38):12550–4.
Liang Y, Zhu Y, Dou M, Xu K, Chu RK, Chrisler WB, et al. Spatially resolved proteome profiling of< 200 cells from tomato fruit pericarp by integrating laser-capture microdissection with nanodroplet sample preparation. Anal Chem. 2018;90(18):11106–14.
Shen Y, Moore RJ, Zhao R, Blonder J, Auberry DL, Masselon C, et al. High-efficiency on-line solid-phase extraction coupling to 15−150-μm-id column liquid chromatography for proteomic analysis. Anal Chem. 2003;75(14):3596–605.
Kelly RT, Page JS, Luo Q, Moore RJ, Orton DJ, Tang K, et al. Chemically etched open tubular and monolithic emitters for nanoelectrospray ionization mass spectrometry. Anal Chem. 2006;78(22):7796–801.
Chen T-C, Webb IK, Prost SA, Harrer MB, Norheim RV, Tang K, et al. Rectangular ion funnel: a new ion funnel interface for structures for lossless ion manipulations. Anal Chem. 2014;87(1):716–22.
Garimella SVB, Hamid AM, Deng L, Ibrahim YM, Webb IK, Baker ES, et al. Squeezing of ion populations and peaks in traveling wave ion mobility separations and structures for lossless ion manipulations using compression ratio ion mobility programming. Anal Chem. 2016;88(23):11877–85.
Deng L, Ibrahim YM, Garimella SVB, Webb IK, Hamid AM, Norheim RV, et al. Greatly increasing trapped ion populations for mobility separations using traveling waves in structures for lossless ion manipulations. Anal Chem. 2016;88(20):10143–50.
Shen Y, Zhao R, Berger SJ, Anderson GA, Rodriguez N, Smith RD. High-efficiency nanoscale liquid chromatography coupled on-line with mass spectrometry using nanoelectrospray ionization for proteomics. Anal Chem. 2002;74(16):4235–49.
Zhou F, Lu Y, Ficarro SB, Webber JT, Marto JA. Nanoflow low pressure high peak capacity single dimension LC-MS/MS platform for high-throughput, in-depth analysis of mammalian proteomes. Anal Chem. 2012;84(11):5133–9.
Wolters DA, Washburn MP, Yates JR. An automated multidimensional protein identification technology for shotgun proteomics. Anal Chem. 2001;73(23):5683–90.
Acknowledgements
This research was performed using EMSL, a national scientific user facility sponsored by the Department of Energy’s Office of Biological and Environmental Research and located at PNNL.
Funding
This work was supported by the NIH grants R21 EB020976 (to R.T.K.) and R33 CA225248 (to R.T.K.) and P41 GM103493 (to R.D.S.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Published in the topical collection Ultrasmall Sample Biochemical Analysis with guest editors Ryan Kelly and Ying Zhu.
Rights and permissions
About this article
Cite this article
Dou, M., Chouinard, C.D., Zhu, Y. et al. Nanowell-mediated multidimensional separations combining nanoLC with SLIM IM-MS for rapid, high-peak-capacity proteomic analyses. Anal Bioanal Chem 411, 5363–5372 (2019). https://doi.org/10.1007/s00216-018-1452-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1452-5