Abstract
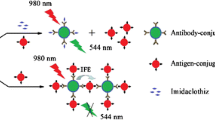
Contamination of the land and water by neonicotinoid insecticide residues is currently a severe environmental problem. However, the traditional methods for pesticide residue analysis are time consuming and laborious. To tackle this problem, here we describe a novel quenchbody (Q-body) immunoassay reagent that allows the rapid and sensitive detection of imidacloprid, one of the most frequently used neonicotinoid pesticides, in aqueous solution. A Q-body comprises an antibody Fab fragment that is site-specifically labeled with a fluorescent dye. The Fab fragment quenches the dye with its internal tryptophan residues via photoinduced electron transfer. The subsequent addition of imidacloprid stabilizes the antibody structure and displaces the quenched dye to the outside of the protein, resulting in increased fluorescence. The constructed Q-body assay exhibited a high dynamic range and a low limit of detection (10 ng mL−1), and the entire assay procedure could be completed in a few minutes. The assay showed a low cross-reactivity with possible interfering analogous compounds, indicating that it has a good selectivity. Hence, the developed Q-body assay has excellent potential as a universal technology for monitoring neonicotinoid residues in environmental and food samples.

A novel quenchbody (Q-body) immunoassay reagent that allows the rapid and sensitive detection of imidacloprid, one of the most frequently used neonicotinoid pesticides, in aqueous solution was developed. The addition of imidacloprid stabilizes the Q-body structure and displaces the quenched dye to the outside of the protein, resulting in increased fluorescence. The constructed Q-body assay exhibited a high dynamic range and a low limit of detection (10 ng mL−1), and completed in a few minutes.



Similar content being viewed by others
References
Tomizawa M, Casida JE. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu Rev Entomol. 2003;48:339–64. https://doi.org/10.1146/annurev.ento.48.091801.112731.
Jeschke P, Nauen R, Schindler M, Elbert A. Overview of the status and global strategy for neonicotinoids. J Agric Food Chem. 2011;59(7):2897–908. https://doi.org/10.1021/jf101303g.
Decourtye A, Devillers J. Ecotoxicity of neonicotinoid insecticides to bees. Adv Exp Med Biol. 2010;683:85–95.
Kessler SC, Tiedeken EJ, Simcock KL, Derveau S, Mitchell J, Softley S, et al. Bees prefer foods containing neonicotinoid pesticides. Nature. 2015;521(7550):74–6. https://doi.org/10.1038/nature14414.
Ruhman MA, Bireley RS, Alder DS. Preliminary pollinator assessment to support the registration review of imidacloprid. Washinton, D.C: US EPA; 2016. https://www.regulations.gov/document?D=EPA-HQ-OPP-2008-0844-0140. Accessed 19 Jan. 2017
Lehotay SJ, de Kok A, Hiemstra M, Van Bodegraven P. Validation of a fast and easy method for the determination of residues from 229 pesticides in fruits and vegetables using gas and liquid chromatography and mass spectrometric detection. J AOAC Int. 2005;88(2):595–614.
Li K, Li QX. Development of an enzyme-linked immunosorbent assay for the insecticide imidacloprid. J Agric Food Chem. 2000;48(8):3378–82.
Lee JK, Ahn KC, Park OS, Kang SY, Hammock BD. Development of an ELISA for the detection of the residues of the insecticide imidacloprid in agricultural and environmental samples. J Agric Food Chem. 2001;49(5):2159–67.
Wang R, Wang Z, Yang H, Wang Y, Deng A. Highly sensitive and specific detection of neonicotinoid insecticide imidacloprid in environmental and food samples by a polyclonal antibody-based enzyme-linked immunosorbent assay. J Sci Food Agric. 2012;92(6):1253–60. https://doi.org/10.1002/jsfa.4691.
Stefano Girotti EM, Ghini S, Eremin S, Manes J. Quantification of imidacloprid in honeybees: development of a chemiluminescent ELISA. Anal Lett. 2010;43(3):466–75.
Xu T, Wei KY, Wang J, Ma HX, Li J, Xu YJ, et al. Quantitative analysis of the neonicotinoid insecticides imidacloprid and thiamethoxam in fruit juices by enzyme-linked immunosorbent assays. J AOAC Int. 2010;93(1):12–8.
Wanatabe S, Ito S, Kamata Y, Omoda N, Yamazaki T, Munakata H, et al. Development of competitive enzyme-linked immunosorbent assays (ELISAs) based on monoclonal antibodies for chloronicotinoid insecticides imidacloprid and acetamiprid. Anal Chim Acta. 2001;427(2):211–9.
Kim H-J, Shelver WL, Li QX. Monoclonal antibody-based enzyme-linked immunosorbent assay for the insecticide imidacloprid. Anal Chim Acta. 2004;509(1):111–8.
Watanabe E, Miyake S, Yogo Y. Review of enzyme-linked immunosorbent assays (ELISAs) for analyses of neonicotinoid insecticides in agro-environments. J Agric Food Chem. 2013;61(51):12459–72. https://doi.org/10.1021/jf403801h.
Abe R, Ohashi H, Iijima I, Ihara M, Takagi H, Hohsaka T, et al. “Quenchbodies”: quench-based antibody probes that show antigen-dependent fluorescence. J Am Chem Soc. 2011;133(43):17386–94. https://doi.org/10.1021/ja205925j.
Abe R, Jeong HJ, Arakawa D, Dong J, Ohashi H, Kaigome R, et al. Ultra Q-bodies: quench-based antibody probes that utilize dye-dye interactions with enhanced antigen-dependent fluorescence. Sci Rep. 2014;4:4640. https://doi.org/10.1038/srep04640.
Jeong HJ, Ohmuro-Matsuyama Y, Ohashi H, Ohsawa F, Tatsu Y, Inagaki M, et al. Detection of vimentin serine phosphorylation by multicolor Quenchbodies. Biosens Bioelectron. 2013;40(1):17–23. https://doi.org/10.1016/j.bios.2012.06.030.
Jeong HJ, Ueda H. Strategy for making a superior Quenchbody to proteins: effect of the fluorophore position. Sensors (Basel). 2014;14(7):13285–97. https://doi.org/10.3390/s140713285.
Ueda H, Dong J. From fluorescence polarization to Quenchbody: recent progress in fluorescent reagentless biosensors based on antibody and other binding proteins. Biochim Biophys Acta. 2014;1844(11):1951–9. https://doi.org/10.1016/j.bbapap.2014.06.005.
Ohashi H, Matsumoto T, Jeong HJ, Dong J, Abe R, Ueda H. Insight into the working mechanism of Quenchbody: transition of the dye around antibody variable region that fluoresces upon antigen binding. Bioconjug Chem. 2016;27(10):2248–53. https://doi.org/10.1021/acs.bioconjchem.6b00217.
Kojima M, Iwai H, Dong J, Lim S-L, Ito S, Okumura K, et al. Activation of circularly permutated β-lactamase tethered to antibody domains by specific small molecules. Bioconjug Chem. 2011;22(4):633–41. https://doi.org/10.1021/bc1004125.
Jeong H-J, Kawamura T, Dong J, Ueda H. Q-bodies from recombinant single-chain Fv fragment with better yield and expanded palette of fluorophores. ACS Sens. 2016;1(1):88–94. https://doi.org/10.1021/acssensors.5b00089.
Ogawa M, Kosaka N, Choyke PL, Kobayashi H. H-type dimer formation of fluorophores: a mechanism for activatable, in vivo optical molecular imaging. ACS Chem Biol. 2009;4(7):535–46. https://doi.org/10.1021/cb900089j.
Kojima M, Iwai H, Dong J, Lim SL, Ito S, Okumura K, et al. Activation of circularly permutated beta-lactamase tethered to antibody domains by specific small molecules. Bioconjug Chem. 2011;22(4):633–41. https://doi.org/10.1021/bc1004125.
The Japan Food Chemical Research Foundation. Maximum residue limits (MRLs) of agricultural chemicals in foods. In: The Japanese positive list system for agricultural chemical residues in foods. Tokyo. 2016. http://www.ffcr.or.jp/zaidan/FFCRHOME.nsf/pages/MRLs-p. Accessed 20 Apr 2018.
Abad A, Moreno MJ, Montoya A. Development of monoclonal antibody-based immunoassays to the N-methylcarbamate pesticide carbofuran. J Agric Food Chem. 1999;47(6):2475–85.
Gui WJ, Liu YH, Wang CM, Liang X, Zhu GN. Development of a direct competitive enzyme-linked immunosorbent assay for parathion residue in food samples. Anal Biochem. 2009;393(1):88–94. https://doi.org/10.1016/j.ab.2009.06.014.
Watanabe E. Review on current analytical methods with chromatographic and nonchromatographic techniques for new generation insecticide neonicotinoids. In: Perveen F, editor. Insecticides—advances in integrated pest management. IntechOpen; 2012. p. 481–510. https://doi.org/10.5772/28032.
Acknowledgements
We thank Chiaki Toyama and Yuki Ohmuro-Matsuyama for their experimental help. We also thank the Biomaterial Analysis Center, Technical Department, Tokyo Institute of Technology for the nucleic acid sequence analysis.
Funding
The authors are partly supported by JSPS KAKENHI (Grant Nos. JP15H04191, JP18H03851 and JP26420793) from the Japan Society for the Promotion of Science; by a Special Developing Research Grant from the Nakatani Foundation, Japan; and by Natural Science Foundation of Shandong Province (Grant Number: ZR2017MB037) and National Natural Science Foundation of China (Grant Number: 21775064).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 597 kb)
Rights and permissions
About this article
Cite this article
Zhao, S., Dong, J., Jeong, HJ. et al. Rapid detection of the neonicotinoid insecticide imidacloprid using a quenchbody assay. Anal Bioanal Chem 410, 4219–4226 (2018). https://doi.org/10.1007/s00216-018-1074-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1074-y