Abstract
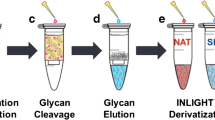
The INLIGHT™ strategy for N-linked glycan derivatization has been shown to overcome many of the challenges associated with glycan analysis. The hydrazide tag reacts efficiently with the glycans, increasing their non-polar surface area, allowing for reversed-phase separations and increased ionization efficiency. We have taken the INLIGHT™ strategy and adopted it for use with O-linked glycans. A central composite design was utilized to find optimized tagging conditions (45% acetic acid, 0.1 μg/μL tag concentration, 37 C, 1.75 h). Derivatization at optimized conditions was much quicker than any hydrazide derivatization strategy used previously. Human immunoglobulin A (IgA) and bovine submaxillary mucin (BSM) were then deglycosylated through hydrazinolysis and the removed glycans were tagged under optimum conditions. XIC of tagged glycans and MS2 data show successful hydrazide tagging of O-linked glycans for the first time.

The INLIGHT™ hydrazide tag was optimized using a central composite design for derivatization of O-linked glycans. Two glycoprotein standards were deglycosylated through hydrazinolysis and tagged at the optimized conditions. MS/MS data shows INLIGHT™ derivatization of glycans demonstrating successful hydrazide tagging of O-glycans for the first time.



Similar content being viewed by others
References
Kudelka MR, Ju T, Heimburg-Molinaro J, Cummings RD. Simple sugars to complex disease—mucin-type O-glycans in cancer. Adv Cancer Res. 2015;126:53–135.
Zauner G, Kozak RP, Gardner RA, Fernandes DL, Deelder AM, Wuhrer M. Protein O-glycosylation analysis. Biol Chem. 2012;393:687–708.
Wuhrer M, Deelder AM, Hokke CH. Protein glycosylation analysis by liquid chromatography-mass spectrometry. J Chromatogr B. 2005;825:124–33.
Kailemia MJ, Ruhaak LR, Lebrilla CB, Amster IJ. Oligosaccharide analysis by mass spectrometry: a review of recent developments. Anal Chem. 2014;86:196–212.
Walker SH, Taylor AD, Muddiman DC. Individual normalization when labeling with isotopic glycan hydrazide tags (INLIGHT): a novel glycan relative quantification strategy. J Am Soc Mass Spectrom. 2013;24:1376–84.
Hecht ES, Scholl EH, Walker SH, Taykir AD, Cliby WA, Motsinger-Reif AA, et al. Relative quantification and higher-order modeling of the plasma glycan cancer burden ratio in ovarian cancer case-control samples. J Proteome Res. 2015;14:4394–401.
Yang S, Yuan W, Yang W, Zhou J, Harlan R, Edwards J, et al. Glycan analysis by isobaric aldehyde reactive tags and mass spectrometry. Anal Chem. 2013;85:8188–95.
Lauber MA, Yu YQ, Brousmiche DW, Hau Z, Koza SM, Magnelli P, et al. Rapid preparation of released N-glycans for HILIC analysis using labeling reagents that facilitates sensitive fluorescence and ESI-MS detection. Anal Chem. 2015;87:5401–9.
Bigge JC, Patel TP, Bruce JA, Goulding PN, Charles SM, Parekh RB. Nonselective and efficient fluorescent labeling of glycans using 2-amino benzamide and anthranilic acid. Anal Biochem. 1995;230:229–38.
Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desportion/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal Biochem. 2007;364:8–18.
Desaire H. Glycopeptide analysis, recent developments and applications. Mol Cell Proteomics. 2013;12:893–901.
Burchell JM, Mungul A, Taylor-Papadimitriou J. O-linked glycosylation in the mammary gland: changes that occur during malignancy. J Mammary Gland Biol Neoplasia. 2001;6:355–64.
Walker SH, Budhathoki-Uprety J, Novak BM, Muddiman DC. Stable-isotope labeled hydrophobic hydrazide reagents for the relative quantification of N-linked glycans by electrospray ionization mass spectrometry. Anal Chem. 2011;83:6738–45.
Mangrum JB, Mehta AY, Alabbas AB, Desai UR, Hawkridge AM. Comparative analysis of INLIGHT™-labeled enzymatically depolymerized heparin by reverse-phase chromatography and high-performance mass spectrometry. Anal Bioanal Chem. 2017;409:499–509.
Hecht ES, Oberg AL, Muddiman DC. Optimizing mass spectrometry analyses: a tailored review on the utility of design of experiments. J Am Soc Mass Spectrom. 2016;27:767–85.
Box GEP, Wilson KB. On the experimental attainment of optimum conditions. J R Stat Soc. 1951;1:1–45.
Fisher RA. An examination of the different possible solutions of a problem in incomplete blocks. Ann Eugenics. 1940;10:52–75.
Iyer RN, Carlson DM. Alkaline borohydride degradation of blood group H substance. Arch Biochem Biophys. 1971;142:101–5.
Yu G, Zhang Y, Zhang Z, Song L, Wang P, Chai W. Effects and limitation of excess ammonium on the release of O-glycans in reducing forms from glycoproteins under mild alkaline conditions for glycomic and functional analysis. Anal Chem. 2010;82:9534–42.
Goetz JA, Novotny MV, Mechref Y. Enzymatic/chemical release of O-glycans allowing MS analysis at high sensitivity. Anal Chem. 2009;81:9546–52.
Haung Y, Mechreg Y, Novotony MV. Microscale nonreductive release of O-linked glycans for subsequent analysis through MALDI mass spectrometry and capillary electrophoresis. Anal Chem. 2001;73:6063–9.
Patel T, Bruce J, Merry A, Bigge C, Wormald M, Jaques A, et al. Use of hydrazine to release in intact and unreduced form both N- and O-linked oligosaccharides from glycoproteins. Biochemistry US. 1993;32:679–93.
Merry AH, Neville DC, Royle L, Matthews B, Harvey DJ, Dwek RA, et al. Recovery of intact 2-aminobenzamide-labeled O-glycans released from glycoproteins by hydrazinolysis. Anal Biochem. 2002;304:91–9.
Hecht ES, McCord JP, Muddiman DC. Definitive screening design optimization of mass spectrometry parameters for sensitive comparison of filter and SPE purified, INLIGHT plasma N-glycans. Anal Chem. 2015;87:7305–12.
Kozak RP, Royle L, Gardner RA, Bondt A, Fernandes DI, Wuhrer M. Improved nonreductive O-glycan release by hydrazinolysis with ethylenediaminetetraacetic acid addition. Anal Biochem. 2014;453:29–37.
Packer NH, Lawsom MA, Jardine DR, Redmond JW. A general approach to desalting oligosaccharides released from glycoproteins. Glycoconj J. 1998;15:737–47.
Loziuk PL, Hecht ES, Muddiman DC. N-linked glycosite profiling and use of skyline as a platform for characterization and relative quantification of glycans in differentiating xylem of Populus trichocarpa. Anal Bioanal Chem. 2017;409:487–97.
Kilcoyne M, Gerlach JQ, Farrell MP, Bhavanandan VP, Joshi L. Periodic acid-Schiff’s reagent assay for carbohydrates in a microtiter plate format. Anal Biochem. 2011;416:18–26.
Acknowledgements
This work was supported by the North Carolina State University Chemistry Graduate Assistantship and the American Chemical Society Division of Analytical Chemistry Fellowship, sponsored by Eli Lilly Inc. The Hydrazinolysis protocol was graciously provided thanks to Dr. Parastoo Azadi at the Complex Carbohydrates Research Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 508 kb)
Rights and permissions
About this article
Cite this article
King, S.R., Hecht, E.S. & Muddiman, D.C. Demonstration of hydrazide tagging for O-glycans and a central composite design of experiments optimization using the INLIGHT™ reagent. Anal Bioanal Chem 410, 1409–1415 (2018). https://doi.org/10.1007/s00216-017-0828-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0828-2