Abstract
Our greater understanding of the importance of N-linked glycosylation in biological systems has spawned the field of glycomics and development of analytical tools to address the many challenges regarding our ability to characterize and quantify this complex and important modification as it relates to biological function. One of the unmet needs of the field remains a systematic method for characterization of glycans in new biological systems. This study presents a novel workflow for identification of glycans using Individuality Normalization when Labeling with Isotopic Glycan Hydrazide Tags (INLIGHT™) strategy developed in our lab. This consists of monoisotopic mass extraction followed by peak pair identification of tagged glycans from a theoretical library using an in-house program. Identification and relative quantification could then be performed using the freely available bioinformatics tool Skyline. These studies were performed in the biological context of studying the N-linked glycome of differentiating xylem of the poplar tree, a widely studied model woody plant, particularly with respect to understanding lignin biosynthesis during wood formation. Through our workflow, we were able to identify 502 glycosylated proteins including 12 monolignol enzymes and 1 peroxidase (PO) through deamidation glycosite analysis. Finally, our novel semi-automated workflow allowed for rapid identification of 27 glycans by intact mass and by NAT/SIL peak pairing from a library containing 1573 potential glycans, eliminating the need for extensive manual analysis. Implementing Skyline for relative glycan quantification allowed for improved accuracy and precision of quantitative measurements over current processing tools which we attribute to superior algorithms correction for baseline variation and MS1 peak filtering.

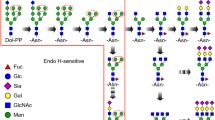
Workflow for FANGS-INLIGHT glycosite profiling of plant xylem and monolignol proteins followed by INLIGHT tagging with semi-automated identification of glycans by light-heavy peak pairs. Finally, manual validation and relative quantification was performed in Skyline




Similar content being viewed by others
References
Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Bba-Gen Subjects. 1999;1:4–8.
Sun S, Zhang H. Identification and validation of atypical N-glycosylation sites. Anal Chem. 2015;24:11948–51.
Lerouge P, Cabanes-Macheteau M, Rayon C, Fischette-Laine AC, Gomord V, Faye L. N-glycoprotein biosynthesis in plants: recent developments and future trends. Plant Mol Biol. 1998;1–2:31–48.
Lukowitz W, Nickle TC, Meinke DW, Last RL, Conklin PL, Somerville CR. Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyltransferase and point to a requirement of N-linked glycosylation for cellulose biosynthesis. Proc Natl Acad Sci U S A. 2001;5:2262–7.
Shuford CM, Li QZ, Sun YH, Chen HC, Wang J, Shi R, et al. Comprehensive quantification of monolignol-pathway enzymes in Populus trichocarpa by protein cleavage isotope dilution mass spectrometry. J Proteome Res. 2012;6:3390–404.
Loziuk PL, Sederoff RR, Chiang VL, Muddiman DC. Establishing ion ratio thresholds based on absolute peak area for absolute protein quantification using protein cleavage isotope dilution mass spectrometry. Analyst. 2014;21:5439–50.
Loziuk PL, Wang J, Li QZ, Sederoff RR, Chiang VL, Muddiman DC. Understanding the role of proteolytic digestion on discovery and targeted proteomic measurements using liquid chromatography tandem mass spectrometry and design of experiments. J Proteome Res. 2013;12:5820–9.
Loziuk PL, Parker J, Li W, Lin CY, Wang JP, Li QZ, et al. Elucidation of xylem-specific transcription factors and absolute quantification of enzymes regulating cellulose biosynthesis in Populus trichocarpa. J Proteome Res. 2015;10:4158–68.
Wang JP, Naik PP, Chen HC, Shi R, Lin CY, Liu J, et al. Complete proteomic-based enzyme reaction and inhibition kinetics reveal how monolignol biosynthetic enzyme families affect metabolic flux and lignin in Populus trichocarpa. Plant Cell. 2014;3:894–914.
Wang JP, Chuang L, Loziuk PL, Chen H, Lin YC, Shi R, et al. Phosphorylation is an on/off switch for 5-hydroxyconiferaldehyde O-methyltransferase activity in poplar monolignol biosynthesis. Proc Natl Acad Sci U S A. 2015;27:8481–6.
Christensen JH, Bauw G, Welinder KG, Van Montagu M, Boerjan W. Purification and characterization of peroxidases correlated with lignification in poplar xylem. Plant Physiol. 1998;1:125–35.
Hecht ES, McCord JP, Muddiman DC. A quantitative glycomics and proteomics combined purification strategy. J Vis Exp. 2016;109, e53735.
Rath CM, Sweeney M, Schmidt B. Analysis of deamidation in monoclonal antibodies: comparing peptide mapping using LC/MS and enzymatic isoaspartate detection using strong cation exchange chromatography. Abstr Pap Am Chem S. 2005;U120-U120.
Tretter V, Altmann F, Marz L. Peptide-N4-(N-acetyl-beta-glucosaminyl)asparagine amidase-F cannot release glycans with fucose attached alpha-1-]3 to the asparagine-linked N-acetylglucosamine residue. Eur J Biochem. 1991;3:647–52.
Hao P, Ren Y, Alpert AJ, Sze SK. Detection, evaluation and minimization of nonenzymatic deamidation in proteomic sample preparation. Mol Cell Proteomics. 2011;10:O111.009381.
Hao P, Ren Y, Datta A, Tam JP, Sze SK. Evaluation of the effect of trypsin digestion buffers on artificial deamidation. J Proteome Res. 2015;2:1308–14.
Robinson NE, Robinson AB. Molecular clocks. Proc Natl Acad Sci U S A. 2001;3:944–9.
Nepomuceno AI, Gibson RJ, Randall SM, Muddiman DC. Accurate identification of deamidated peptides in global proteomics using a quadrupole orbitrap mass spectrometer. J Proteome Res. 2014;2:777–85.
Gonzalez J, Takao T, Hori H, Besada V, Rodriguez R, Padron G, et al. A method for determination of N-glycosylation sites in glycoproteins by collision-induced dissociation analysis in fast-atom-bombardment mass-spectrometry—identification of the positions of carbohydrate-linked asparagine in recombinant alpha-amylase by treatment with peptide-N-glycosidase-F in O-18-labeled. Water Anal Biochem. 1992;1:151–8.
Shuford CM, Muddiman DC. Capitalizing on the hydrophobic bias of electrospray ionization through chemical modification in mass spectrometry-based proteomics. Expert Rev Proteomics. 2011;3:317–23.
Walker SH, Taylor AD, Muddiman DC. Individuality normalization when labeling with isotopic glycan hydrazide tags (INLIGHT): a novel glycan-relative quantification strategy. J Am Soc Mass Spectr. 2013;9:1376–84.
Hecht ES, McCord JP, Muddiman DC. Definitive screening design optimization of mass spectrometry parameters for sensitive comparison of filter and solid phase extraction purified, INLIGHT plasma N-glycans. Anal Chem. 2015;14:7305–12.
Meitei NS, Apte A, Snovida SI, Rogers JC, Saba J. Automating mass spectrometry-based quantitative glycomics using aminoxy tandem mass tag reagents with SimGlycan. J Proteomics. 2015;211–222.
Peng B, Ahrends R. Adaptation of Skyline for targeted lipidomics. J Proteome Res. 2016;1:291–301.
Liu SS, Chen X, Yan ZH, Qin SS, Xu JH, Lin JP, et al. Exploring Skyline for both MSE-based label-free proteomics and HRMS quantitation of small molecules. Proteomics. 2014;2–3:169–80.
Shi R, Sun YH, Li Q, Heber S, Sederoff R, Chiang VL. Towards a systems approach for lignin biosynthesis in Populus trichocarpa: transcript abundance and specificity of the monolignol biosynthetic genes. Plant Cell Physiol. 2010;1:144–63.
Hecht ES, Scholl EH, Walker SH, Taylor AD, Cliby WA, Motsinger-Reif AA, et al. Relative quantification and higher-order modeling of the plasma glycan cancer burden ratio in ovarian cancer case–control samples. J Proteome Res. 2015;10:4394–401.
Holman JD, Tabb DL, Mallick P. Employing ProteoWizard to convert raw mass spectrometry data. Curr Protoc Bioinform. 2014;13(24):11–9.
Hoopmann MR, Finney GL, MacCoss MJ. High-speed data reduction, feature detection and MS/MS spectrum quality assessment of shotgun proteomics data sets using high-resolution mass spectrometry. Anal Chem. 2007;15:5620–32.
Hoopmann MR, MacCoss MJ, Moritz RL. Identification of peptide features in precursor spectra using Hardklor and Kronik. Curr Protoc Bioinformatics. 2012;Unit13 18.
MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;7:966–8.
Djerbi S, Lindskog M, Arvestad L, Sterky F, Teeri TT. The genome sequence of black cottonwood (Populus trichocarpa) reveals 18 conserved cellulose synthase (CesA) genes. Planta. 2005;5:739–46.
Chou MF, Schwartz D. Biological sequence motif discovery using motif-x. Curr Protoc Bioinformatics Unit. 2011;13:15–24.
Schwartz D, Chou MF, Church GM. Predicting protein post-translational modifications using meta-analysis of proteome scale data sets. Mol Cell Proteomics. 2009;2:365–79.
Huang DW, Sherman BT, Tan Q, Collins JR, Alvord WG, Roayaei J, et al. The DAVID gene functional classification tool: a novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007;9:R183.
Lin CY, Li QZ, Tunlaya-Anukit S, Shi R, Sun YH, Wang JP, Liu J, Loziuk P, Edmunds CW, Miller ZD et al. A cell wall-bound anionic peroxidase, PtrPO21, is involved in lignin polymerization in Populus trichocarpa. Tree Genet Genomes. 2016;2.
Glycan Structures Database. In. CFG: Functional Glycomics Gateway: Consortium for Functional Glycomics. 2016.
Woodward JR, Craik D, Dell A, Khoo K-H, Munro SLA, Clarke AE, et al. Structural analysis of the N-linked glycan chains from a stylar glycoprotein associated with expression of self-incompatibility in Nicotiana alata. Glycobiology. 1992;3:241–50.
Oxley D, Bacic A. Microheterogeneity of N-glycosylation on a stylar self-incompatibility glycoprotein of Nicotiana alata. Glycobiology. 1995;5:517–23.
Lommerse JPM, Thomas-Oates JE, Gielens C, Préaux G, Kamerling JP, Vliegenthart JFG. Primary structure of 21 novel monoantennary and diantennary N-linked carbohydrate chains from αD-hemocyanin of helix pomatia. Eur J Biochem. 1997;1:195–222.
Aguilera F, McDougall C, Degnan BM. Origin, evolution and classification of type-3 copper proteins: lineage-specific gene expansions and losses across the Metazoa. BMC Evol Biol. 2013;1:1–12.
Riboflavin metabolism. In. http://www.genome.jp/kegg-bin/show_pathway?org_name=pop&mapno=00740&mapscale=&show_description=hide: KEGG Pathway 00740; 2015.
Isoquinoline alkaloid biosynthesis. In. http://www.genome.jp/kegg-bin/show_pathway?org_name=pop&mapno=00950&mapscale=&show_description=hide: KEGG Pathway 00950; 2014.
Ro DK, Mah N, Ellis BE, Douglas CJ. Functional characterization and subcellular localization of poplar (Populus trichocarpa x Populus deltoides) cinnamate 4-hydroxylase. Plant Physiol. 2001;1:317–29.
Hoffmann L, Besseau S, Geoffroy P, Ritzenthaler C, Meyer D, Lapierre C, et al. Silencing of hydroxycinnamoyl-coenzyme A shikimate/quinate hydroxycinnamoyltransferase affects phenylpropanoid biosynthesis. Plant Cell. 2004;6:1446–65.
Ceriotti A, Duranti M, Bollini R. Effects of N-glycosylation on the folding and structure of plant proteins. J Exp Bot. 1998;324:1091–103.
Chen HC, Li QZ, Shuford CM, Liu J, Muddiman DC, Sederoff RR, et al. Membrane protein complexes catalyze both 4- and 3-hydroxylation of cinnamic acid derivatives in monolignol biosynthesis. Proc Natl Acad Sci U S A. 2011;52:21253–8.
Acknowledgments
This material is based on work supported by North Carolina State University and the Chemistry Graduate Assistantship and the NIH/NCSU Molecular Biotechnology Training Program (Grant 5T32GM00-8776-08). We gratefully acknowledge Jack P. Wang and Vincent L. Chiang for providing the xylem used in this study. Finally, we would also like to thank Jon Ziefle for writing MIsearch.py.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest in the present work.
Additional information
Published in the topical collection Glycomics, Glycoproteomics and Allied Topics with guest editors Yehia Mechref and David Muddiman.
Rights and permissions
About this article
Cite this article
Loziuk, P.L., Hecht, E.S. & Muddiman, D.C. N-linked glycosite profiling and use of Skyline as a platform for characterization and relative quantification of glycans in differentiating xylem of Populus trichocarpa . Anal Bioanal Chem 409, 487–497 (2017). https://doi.org/10.1007/s00216-016-9776-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9776-5