Abstract
Rationale
Genetic and environmental factors cause neuropsychiatric disorders through complex interactions that are far from understood. Loss-of-function mutations in synaptic proteins like neurexin1α have been linked to autism spectrum disorders (ASD) and schizophrenia (SCZ), both characterised by problems in social behaviour. Childhood social play behaviour is thought to facilitate social development, and lack of social play may precipitate or exacerbate ASD and SCZ.
Objective
To test the hypothesis that an environmental insult acts on top of genetic vulnerability to precipitate psychiatric-like phenotypes. To that aim, social behaviour in neurexin1α knockout rats was assessed, with or without deprivation of juvenile social play. We also tested drugs prescribed in ASD or SCZ to assess the relevance of this dual-hit model for these disorders.
Results
Neurexin1α knockout rats showed an aberrant social phenotype, with high amounts of social play, increased motivation to play, age-inappropriate sexual mounting, and an increase in general activity. Play deprivation subtly altered later social behaviour, but did not affect the phenotype of neurexin1α knockout rats. Risperidone and methylphenidate decreased play behaviour in both wild-type and knockout rats. Amphetamine-induced hyperactivity was exaggerated in neurexin1α knockout rats.
Conclusion
Deletion of the neurexin1α gene in rats causes exaggerated social play, which is not modified by social play deprivation. This phenotype therefore resembles disinhibited behaviour rather than the social withdrawal seen in ASD and SCZ. The neurexin1α knockout rat could be a model for inappropriate or disinhibited social behaviour seen in childhood mental disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genetics and environmental factors interact in a complex fashion to cause, precipitate or exacerbate mental disorders, but the underlying mechanisms remain elusive. Moreover, only a proportion of individuals that exhibit prodromal signs, such as social withdrawal, ultimately develop true psychiatric symptoms, likely due to this combination of genetic predisposition and environmental factors (Shields 1980). Schizophrenia (SCZ) and autism spectrum disorders (ASD) are neurodevelopmental disorders with strong genetic components, but also characterised by very diverse clinical presentations despite shared genetics. This is most clearly illustrated in monozygotic twins, in which, although concordance rates are higher, differences still exist that can only be explained by (early) life factors that change the course of the disease (Gejman et al. 2010; Imamura et al. 2020). Genetic analyses have revealed a multitude of candidate vulnerability genes, many of which code for synaptic proteins. Of these, the neurexin-1 (nrxn1) gene has been linked directly to ASD and SCZ (Kim et al. 2008; Rujescu et al. 2009, Glessner et al. 2009; Ching et al. 2010; Gauthier et al. 2011). The neurexin gene family consists of three members (neurexin1–3) that encode presynaptic cell adhesion proteins that form trans-synaptic complexes with postsynaptic neuroligins. These neurexin-neuroligin complexes are thought to mediate synaptic signalling, specification and maintenance, and to control the balance of inhibitory GABAergic and excitatory glutamatergic neurotransmission (Südhof 2008). Importantly, alterations in the balance between excitatory and inhibitory signalling in neural micro-circuitry have been implicated in social deficits as seen in ASD and SCZ (Yizhar et al. 2011).
To investigate the causative role of nrxn1 in ASD- and SCZ-like phenotypes, animal models with a genetic ablation have been created. In mice, ablation of nrxn1 resulted in detrimental effects on prepulse inhibition and spatial memory, and increased grooming and motor learning (Etherton et al. 2009; Blundell et al. 2010), but in the social spectrum, only mild phenotypes and varying results have been described (Grayton et al. 2013, Rabaneda et al. 2014, Dachtler et al. 2015; Armstrong et al. 2020). To specifically explore the role of nrxn1 deletion on social behaviour throughout development, rats are preferrable to study because their complex social behavioural repertoire, and especially the characteristic display of play behaviour early in development. Previous studies with nrxn1-deficient rats have shown profound impairments in attention and cognitive capacities, as well as hyperactivity (Esclassan et al. 2015), altered patterns of social interest (Twining et al. 2017), sex-specific deficits in ultrasonic vocalizations and social play, but unaffected sociability and social discrimination (Kight et al. 2021). Janz et al. (2022) reported hyperactivity, deficits in context-dependent auditory processing but a normal response towards social stimuli. Recently, increased social interest, as well as reduced anxiety in the open field, was reported in rats in which the nrxn1 gene was down-regulated in the medial prefrontal cortex (Wu et al. 2023).
Social dysfunction, which has an enormous impact on everyday life, is a hallmark of a ASD and SCZ. These social deficits typically remain untreated, mostly because the underlying neural mechanisms are poorly understood. Importantly, ASD and SCZ manifest already in childhood or adolescence, indicating that the development of the social brain is a process vulnerable to early disturbances. During early life, social interactions typically take the form of social play behaviour, which is abundant in the young of most mammalian species, including rats and humans. Alongside its pleasurable properties, social play is thought to contribute to the development of brain and behaviour (Panksepp et al. 1984; Graham and Burghardt 2010; Vanderschuren and Trezza 2014; Pellis et al. 2023). Indeed, depriving rats of social play behaviour during development results in impairments in the social, emotional and cognitive domains (Vanderschuren and Trezza 2014; Pellis et al. 2023). With regard to human psychopathology, social withdrawal is a prominent negative symptom of SCZ (Marder and Galderisi 2017). A preference for solitary play in childhood seems to predict SCZ (Helgeland and Torgersen 2005; Jones et al. 1994) and play behaviour is disrupted during the prodromal phase of SCZ (Møller and Husby 2000). Furthermore, play has been shown to be impaired in children with ASD (Jarrold 2003; Manning and Wainwright 2010) and impaired social play in ASD is thought to intensify the disorder (Jordan 2003).
To further understand how genetics and the early social environment interact to worsen ASD- or SCZ-like symptomatology, in the present study we used deletion of the neurexin1α gene and lack of social play as the genetic vulnerability and environment insult, respectively. We hypothesize that decreased social interaction the juvenile period acts on top of a genetic vulnerability to precipitate abnormal social behaviour in adolescence and adulthood. Specific aims were: 1. to investigate juvenile, adolescent and adult social (play) behaviour of neurexin1α-knockout (nrxn1-KO) rats; 2. to identify neuropharmacological correlates of the altered behaviour in nrxn1-KO rats, by testing risperidone and methylphenidate, drugs commonly used for symptom management in ASD and SCZ, as well as amphetamine, that exacerbates symptoms of SCZ; 3. to establish a ‘dual-hit’ model for ASD/SCZ, by subjecting nrxn1-KO rats to deprivation of social play behaviour.
Materials and methods
Animals and housing conditions
Male and female neurexin1α-knockout (nrxn1-KO) rats and wild type (WT) control rats (Horizon Discovery, Boyertown, PA) were bred in the animal facilities at Utrecht University and the Roche Innovation Center Basel. Rats were homozygous knockouts, containing a bi-allelic deletion of the nrxn1α gene on a Sprague Dawley background. WT and nrxn1-KO rats were bred from heterozygous pairs. Pups were toe-clipped on post-natal day (PND) 7-9 or ear-punched on PND14 for genotypic validation and weaned between PND21-24. Animals were housed with same-genotype and same-sex cagemates in temperature-controlled rooms (20-21°C, 60-65% relative humidity) in Macrolon cages with ad libitum access to food and water, under a 12:12-h light–dark schedule (lights on at 7.00 a.m.). All experiments were performed during the light phase of the day-night cycle. All experiments were approved by the Animal Ethics Committee of Utrecht University and the Kantonal Experimentation Committee and were conducted in accordance with Dutch (Wet op Dierproeven, 1996), Swiss (Federal Act on Animal Protection; 1978) and European legislation (Guideline 86/609/EEC; Directive 2010/63/EU). For an overview of the cohorts of animals and the experiments in which they were used, see Table 1.
To deprive the rats of social play, they were socially isolated during PND21-42, i.e. the period in life when social play behaviour is most abundant (Baenninger 1967; Meaney and Stewart 1981; Panksepp 1981). We have previously shown that depriving rats of social play during this period in life causes long-lasting deficits in executive functions (Baarendse et al. 2013; Bijlsma et al. 2022), and enhances the sensitivity to substance use (Baarendse et al. 2014; Lesscher et al. 2015). On PND21 ± 3 days, half of the animals were socially housed in pairs or triads. The other half of the animals were housed in pairs, but a transparent Plexiglas divider containing small holes was placed in the middle of the home cage, creating two separate but identical compartments. Through these dividers, animals were able to exchange visual, olfactory and auditory information but physically engaging in social play behaviour was prevented. On P42, the Plexiglas divider was removed and all rats were housed in pairs for the remainder of the experiment. In cohorts 1 and 3, the animals were tested for social play after 24 hrs of resocialization. In cohort 4, animals were tested after 4 days of resocialization for social play. Cohorts 1 and 3 were again tested for social behaviour and locomotor activity in adulthood (at 11 weeks of age and onwards, see below).
Drugs
Risperidone hydrochloride (0-0.06-0.2 mg/kg) was administered 1 hour before, and methylphenidate hydrochloride (0-0.3-1.0 mg/kg) was given 30 minutes before testing for social play (see below). d-Amphetamine sulphate (0-0.25-0.5 mg/kg) was administered after 30 min habituation in the locomotor chambers. All drugs were synthetised in-house at the Roche Innovation Center Basel, and administered subcutaneously. Drug doses were carefully selected based on previous research (Vanderschuren et al. 2008; Achterberg et al. 2014; Roche in house data), and doses were calculated as salt. In view of the importance of the neck area in the expression of social play behaviour (Pellis and Pellis 1987; Siviy and Panksepp 1987), subcutaneous injections were administered in the flank.
Social play behaviour
The procedures for social play analysis were as previously described (Vanderschuren et al. 2008). Social play behaviour in rats first emerges around weaning (PND21), peaks between PND28-35 and declines after the onset of puberty (around PND42), and low levels of play can still be observed in adult rats (Meaney and Stewart 1981; Panksepp 1981). Therefore, to capture the developmental time course of social play, nrxn1-KO and WT rats were tested for social play behaviour three times, i.e. at PND21-24 (W4, week 4), PND45-49 (W7, week 7) and PND 75-77 (W11, week 11) weeks of age. The same pairs of animals (see below) were tested after 4 and 7 weeks, and a subset of these animals was tested at 11 weeks. The effects of risperidone and methylphenidate on social play behaviour were tested in a separate cohort of animals, at PND27-28 (risperidone) and PND40-42 (methylphenidate) of age. This cohort consisted only of male rats due to the capacity of facility.
The experiments were performed in a sound attenuated chamber under red light conditions. Animals were paired with an unfamiliar partner (i.e., not a cage mate) of the same genotype, and animals in a test pair did not differ more than 10 g in body weight. The testing arena consisted of a Plexiglas cage measuring 40×40×60 cm (l × w × h), with approximately 2 cm of wood shavings covering the floor. On the two days before testing, the rats were individually habituated to the test cage for 10 min. On the test day, the animals were socially isolated for 2.5 h before testing. The test consisted of placing two animals into the test cage for 15 min. The behaviour of the animals was digitally recorded and scored live by an observer blind to genotype and housing condition. Behaviour was assessed per pair of animals or individually, depending on the experiment, using Observer 5.1 software (Noldus Information Technology BV, Wageningen, The Netherlands).
In rats, a bout of social play behaviour starts with one rat soliciting another animal to play by pouncing, i.e. touching the nape of the neck of the other animal with its snout. If the animal that is pounced upon can fully rotates to its dorsal surface, “pinning” is the result. From this position, the supine animal can initiate another play bout, by trying to gain access to the other animal’s neck. Thus, during social play, pouncing is considered an index of play solicitation, while pinning functions as a releaser of a prolonged play bout (Panksepp and Beatty 1980; Pellis and Pellis 1987; Poole and Fish 1975). Pinning and pouncing frequencies are considered the most characteristic parameters of social play behaviour in rats (Panksepp and Beatty 1980; Trezza et al. 2010). The following behaviours were therefore scored:
-
Frequency of pinning: one animal lying with its dorsal surface on the floor with the other animal standing over it.
-
Frequency of pouncing: one animal attempting to nose or rub the nape of the neck of the other animal.
-
Duration of social exploration: one animal sniffing or grooming any part of the partner’s body
-
Duration of non-social exploration: moving around in (walking or rearing) or sniffing any part of the test cage.
Responding for social play
Behavioural testing was conducted in an operant conditioning chamber (Med Associates, Georgia, VT, USA) divided into two equally sized compartments (25 × 30 × 25 cm, l × w × h). The compartments were separated by a Plexiglas wall with 42 small holes (Ø0.5 cm) and an automated metal door in the middle. Both compartments had a metal grid floor and a Plexiglas lid which contained a house-light (2 W). One compartment was equipped with two 4.8 cm-wide retractable levers, located on opposite sides of the compartment. Above each lever was a cue light (2.5 W). One lever was designated as the active lever and the other as the inactive lever; allocation of the left or right lever as active was counterbalanced between animals, but kept constant for individual animals. Experimental events and data recording were controlled using Med-PC software (Med Associates, Georgia, VT, USA).
Operant conditioning was performed as previously described (Achterberg et al. 2016a, b). All experiments were performed under red light conditions. In this experiment, only pairs of male rats were tested due to capacity of the equipment and the facility. Rats were trained from PND24, and tested between PND37-50. A test pair consisted of one experimental animal and one unfamiliar stimulus partner of the same genotype. Rats within a test pair did not differ more than 10 g in body weight at the start of the experiment. On PND 24, test pairs were habituated to the test cage for 10 min. After the habituation session, the animals were socially isolated for 24 h/day for 5 consecutive days per week. Next, the animals received two shaping sessions on two consecutive days. During these shaping sessions, the cue light was presented, the lever retracted and the door opened whenever the experimental animal approached the active lever, after which the rats were allowed to interact for two minutes. This procedure was repeated 7 times. If an animal did not perform any active lever presses during acquisition sessions, it received an additional shaping session later that day. On the fourth day, the lever pressing sessions (20 min) commenced under a fixed ratio (FR)-1 schedule of reinforcement, under which each active lever press resulted in presentation of the cue light, retraction of both levers, and opening of the door, after which animals were allowed to freely interact for 2 min. After acquisition of the task under the FR-1 schedule (i.e., when an animal obtained at least six out of eight possible rewards on two consecutive days), a progressive ratio (PR) schedule of reinforcement was introduced. Under this schedule, the animals had to meet a response requirement on the active lever that progressively increased after every earned reward (1, 2, 4, 6, 9, 12, 15, 25, etc; Hodos 1961; Richardson and Roberts 1996). When rats met the response requirement, the cue light was illuminated, both levers retracted and the door opened for 1 min, during which the animals could freely interact. Inactive lever presses were recorded, but had no programmed consequences. A PR session continued until an animal failed to obtain a reward within 10 min. Animals received one session per day, for 5 consecutive days per week. During the other 2 days/week animals were socially housed with their original cage-mates.
After responding had stabilized, defined as obtaining at least six rewards on three consecutive days with a variation of no more than two rewards, rats were tested with a same-age same genotype unfamiliar partner. During the earned 1 min social interactions, behaviour of the rats was assessed on-line using the Observer 5.1 software (Noldus Information Technology B.V., The Netherlands). In addition to the on-line analysis, behaviour of the animals was recorded using a camera with zoom lens, video tape recorder and television monitor. Three behavioural elements were scored, i.e. frequency of pinning, frequency of pouncing, and duration of social and non-social exploration. As the accumulated time spent in play sessions varies depending on the amount of obtained rewards, frequencies and durations are expressed per minute.
Adult social behaviour
Experiments were conducted when the animals were 11 weeks old (PND75-77), in the same boxes as described for the analysis of social play behaviour (see above). The animals were habituated to the test cages for 10 minutes on two consecutive days prior to testing. Animals were socially isolated for 24 hours before testing to enhance the motivation to engage in social interaction (Niesink and Van Ree 1982). A test pair consisted of unfamiliar animals of the same genotype, housing condition and sex and they did not differ by more than 25 grams in bodyweight. The test consisted of placing two animals into the test cage for 15 min. Behaviour was recorded and assessed as described above (see Social play behaviour). The following parameters were scored per test pair or individually.
Social play behaviour (see 2.3):
-
Frequency of pinning
-
Frequency of pouncing
Non-playful social behaviour:
-
Frequency of sexual mounting
-
Duration of social exploration (sniffing/grooming/licking any part of the body of the other rat, excluding the anogenital area)
-
Duration of anogenital investigation (sniffing the anogenital area of the other rat)
-
Duration of following or chasing (moving or running forward in the direction of or pursuing the other rat, who moves away)
Agonistic social behaviour :
-
Frequency of boxing (rearing in an upright position towards the other rat, combined with both rats rapidly pushing, pawing and grabbing at each other, or one rat wrapping around the other subject)
-
Frequency of kicking (one animal extends its hind paw to the other animal to keep it away from contacting the body).
Cage exploration:
Duration of non-social exploration
Social interest: three chamber test
The experiment was conducted as described in Kentrop et al. (2018). The three-chamber arena (120 cm x 80 cm x 40 cm) consisted of a black acrylic floor and transparent acrylic walls that separate the arena into three equally sized compartments (Sociability cage for rats, Noldus Information Technology B.V., Wageningen, The Netherlands). The walls of the middle compartment contained an opening to the outer compartments that could be closed with removable slide doors. Two cylinders with a diameter of 22 cm (40 cm in height) were placed in the outer compartments to contain stimulus rats during testing. These cylinders were made of acrylic bars placed 15 mm apart to allow for close contact while preventing physical interaction. All three-chamber experiments were performed in dim light conditions (10 lux). Between tests, the arena and cylinders were cleaned with a 0.5 % v/v solution of Shureclean VK 10 (Johnson Diversey, United Kingdom) dissolved in warm water.
Animals were individually habituated to the arena without the cylinders in two 5-min habituation sessions, the week before testing. Unfamiliar, sex- and age-matched nrxn1 heterozygous rats were used as stimulus partners. The stimulus partners were habituated twice for 5 min to the cylinders, two days prior to testing. They were used for a maximum of three tests per day separated at least by one hour.
The experimental procedure consisted of three phases. Between phases, the test rat was confined in the middle compartment for approximately 1 min.
-
Phase I: Habituation (5 min). The subject was placed in the middle compartment and the sliding doors that provided access to the outer compartments were removed. The rat was allowed to freely explore all three compartments and the two cylinders that were placed in the outer compartments. In this phase the cylinders were empty, but unfamiliar and therefore represented novel objects.
-
Phase II: Social interest (10 min): A stimulus rat was placed in one of the cylinders, while the other cylinder remained empty. Again, both sliding doors were removed and the test rat had access to all compartments.
-
Phase III: Social discrimination (10 min): The stimulus rat from phase II was now considered familiar. The familiar stimulus rat remained in the cylinder and a second (novel) stimulus rat was placed in the other cylinder. Both doors were opened and the test rat was allowed to freely move around the arena for 10 min.
Ethovision XT11 (Noldus Information and Technology BV, Wageningen, The Netherlands) was used to track the position of the rats in the arena. In addition to the three chamber zones, two ‘interaction’ zones, i.e. a 10 cm perimeter around the cylinders were defined. Analysed parameters included time spent in the different compartments and time spent in the interaction zones. The proportion of interaction time was calculated to assess the preference of the test rat for one of the cylinders: (time spent in the interaction zone containing the unfamiliar stimulus rat (social interest phase) or the new unfamiliar stimulus rat (social discrimination phase) / the total time spent in both interaction zones.
Locomotor activity
Testing for horizontal locomotor activity was performed as previously described (Veeneman et al. 2011). Rats were transferred to a plastic cage (l x w x h, 50 x 33 x 40 cm) and their position was tracked five times per second for 30 min using a video-tracking system (EthoVision, Noldus Information Technology, The Netherlands). This was done twice, i.e. at 6 weeks (PND38-40, adolescence) and 11 weeks of age (PND75-77, adulthood) in a subset of the same individuals. The locomotor response to treatment with d-amphetamine was assessed in adult male rats (15 weeks of age): the animals were habituated to the apparatus (l x w x h, 40 x 40 x 30 cm, VersaMax Animal Activity Monitoring System, AccuScan Instruments, Columbus, USA) for 30 min, after which they received an injection with amphetamine (0, 0.25 or 0.5 mg/kg, s.c.), were directly put back into the apparatus and activity was recorded for an additional 60 min.
Statistical analysis
Data was analysed using SPSS software 24 for Windows (IBM, United States) and expressed as mean + SEM. Social play and adult social behaviour were analysed using 2- or 3-way ANOVAs with genotype (WT or nrxn1-KO), housing condition (socially housed or play-deprived) and sex as between-subject factors. The effects of methylphenidate and risperidone on social play were analysed using 2-way ANOVA with genotype and treatment as between-subject factors. Responding for social play was analysed using a 2-way repeated measures ANOVA with lever (active or inactive) as the within-subject factor and genotype as the between-subject factor. The number of obtained rewards, pinning frequency, pouncing frequency and the amount of social exploration during reinforced trials were analysed using one-way ANOVA with genotype as between subject factor. Breakpoints under the PR schedule of reinforcement, i.e., the highest number of lever presses made for a single reward in a session, are derived from an escalating curve, which violates the homogeneity of variance. Therefore, breakpoints were analysed using the non-parametric Friedman test, followed by a post hoc Wilcoxon signed ranks test when appropriate. For the three-chamber test, the proportion of interaction time was analysed with a one sample Student’s t-test against 50% chance level. In addition, a between-groups analysis of the proportion of interaction time was performed using 3-way ANOVA with genotype, housing and sex as between-subject factors. Locomotor activity was expressed as distance moved in 5 min bins and was analysed using a 3-way repeated-measures ANOVA with genotype, housing and sex as between-subject factors. The effect of amphetamine on locomotion was analysed using a repeated-measures ANOVA with time as within-subject factor and genotype, housing and dose as between-subject factors. Where appropriate, post hoc analysis was performed using one-way ANOVAs and paired or unpaired Students T-tests with Bonferroni correction.
Results
Social play behaviour in nrxn1-KO and WT rats
To capture the developmental time course of social play, rats were tested at 4, 7 and 11 weeks of age. Because there were no differences between male and female rats, the data were pooled. At week 4, nrxn1-KO rats pinned and pounced more than WT rats (tpouncing(32)=4.01, p<0.001 Fig. 1A; tpinning(32)=5.00, p<0.001, Fig. 1B) whereas social exploration was reduced (tsocialexploration(32)=4.70, p<0.001, Fig. 1C). Non-social exploration was similar in the two genotypes (tnonsocialexploration(32)=1.70, p=0.10, Fig. 1D). Unexpectedly, the nrxn1-KO rats displayed sexual mounting behaviour during play, which was nearly absent in WT (WT, in 1 out of 18 pairs; nrxn1-KO, in 10 out of 16 pairs; tmounting(32)=3.18, p=0.003, Fig 1E).
Social play behaviour in nrxn1-KO (grey bars) and WT (white bars) rats. Pouncing (A), pinning (B), social exploration (C), non-social exploration (D) and sexual mounting behaviour (E) at 4 weeks (W4, PND21-24), 7 weeks (W7, PND45-49) and 11 weeks of age (W11, PND75-77) in nrxn1-KO and WT rats. Data presented as mean + SEM: *p<0.05, ***p<0.001, #p<0.09. W4 and W7: WT: n=18, KO: n=16 pairs. W11: WT: n=10, KO: n=9 pairs
Also at W7, the nrxn1-KO rats displayed more social play behaviour than WT rats (Fig. 1A and 1B; tpouncing(32)=2.60, p=0.01; tpinning(32)=2.15, p=0.04). Social exploration was reduced in the nrxn1-KO rats (tsocialexploration(32)=4.97, p<0.001, Fig. 1C) whereas non-social exploration did not differ between nrxn1-KO and WT animals (tnonsocialexploration(32)=0.34, p=0.73, Fig. 1D). The nrxn1-KO rats also showed higher levels of sexual mounting than WT (WT, in 4 out of 18 pairs; KO, in 13 out of 16 pairs; tmounting(32)=3.89, p<0.001, Fig 1E).
At W11, the nrxn1-KO rats pounced more (tpouncing(17)=2.55, p=0.02) and tended to pin more (tpinning(17)=1.83, p=0.09, Fig. 1A,B) than WT rats whereas the amount time spent on social exploration was similar between the genotypes (tsocialexploration(17)=0.56, p=0.59, Fig 1C). The time spent on non-social exploration tended to be lower in nrxn1-KO animals (tnonsocialexploration(17)=1.89, p=0.08, Fig 1D). Comparable to W4 and W7, the nrxn1-KO rats but not the WT showed sexual mounting during play (WT, in 0 out of 10 pairs; KO, in 6 out of 9 pairs; tmounting(32)=2.92, p=0.001, Fig 1E).
Responding for social play behaviour in nrxn1-KO and WT rats
Male rats of both genotypes learned to perform the task, as they differentiated between the levers (Flever(1,7)= 29.23, p=0.001), pressing the active lever significantly more than the inactive lever. The performance on the levers differed between genotypes (Fgenotype(1,7)=8.54, p=0.02; Flever*genotype(1,7)=9.35, p=0.02). When investigating performance on the active an inactive levers separately, nrxn1-KO rats made significantly more active responses than WT rats (t(7)=-3.14, p=0.02, Fig. 2A) whereas responding on the inactive lever was similar in both genotypes (t(7)=-0.74, p=0.48, Fig. 2A). Furthermore, nrxn1-KO rats collected more rewards than WT rats (t(7)=-3.65, p=0.01, Fig. 2B) and the breakpoint was higher (Z=-2.46, p=0.01, Fig. 2C). These results indicate a higher motivation for social play in nrxn1-KO rats.
Operant responding for social play behaviour in nrxn1-KO (grey bars) and WT (white bars) rats. A. The amount of lever presses on the active (left panel) versus the inactive (right panel) lever. B. Number of rewards collected during the session. C. Breakpoint of responding for social play. D-E-F: Frequency of pouncing (D) and pinning (E) and percentage of time spent in social and non-social exploration (F) during the reinforced trials. Data are expressed as mean + SEM. *p<0.05 nrxn1-KO compared to WT, @@@ p<0.001 and $$$ p<0.001 compared to active lever-presses of the same genotype. WT: n=5 pairs, nrxn1-KO: n=4 pairs
Social play behaviour during reinforced trials, assessed as pounces and pins per minute, did not differ between the genotypes (pouncing: t(7)=0.20, p=0.85; pinning: t(7)=0.28, p=0.79; 2D and E). Social as well as non-social exploration in this task was also comparable in nrxn1-KO and WT rats (social exploration: t(7)= 1.10, p=0.31; non-social exploration: t(7)=-0.71, p=0.50, 2F).
Effects of risperidone and methylphenidate on social play behaviour in nrxn1-KO and WT rats
Risperidone (0-0.06-0.2 mg/kg) affected social play behaviour in male WT and nrxn1-KO rats similarly (pouncing: Fgenotype*treatment(2,67)=0.36, p=0.70; pinning: Fgenotype*treatment(2,67)=0.58, p=0.56; social exploration: Fgenotype*treatment(2,67)=0.44, p=0.65) to reduce both pouncing (Ftreatment(2,67)= 14.60, p<0.001, post hoc veh vs 0.06 mg/kg: p=0.002; veh vs 0.2mg/kg: p<0.001; 0.06 vs 0.2 mg/kg: p=0.30, 3A) and pinning (Ftreatment(2,67)=13.45, p<0.001, post hoc veh vs 0.06 mg/kg: p<0.001; veh vs 0.2mg/kg: p<0.001; 0.06 vs 0.2 mg/kg: p=0.99, 3B) at both doses tested, but not social exploratory behaviour (Ftreatment(2,67)=2.57, p=0.08, 3C). Risperidone affected non-social exploration differently in the two genotypes (Fgenotype*treatment(2,67)=3.23, p=0.05; Ftreatment(2,67)=11.73, p<0.001). Non-social exploration was enhanced after treatment with the low but not the higher dose of risperidone in WT rats whereas it was enhanced by both doses in KO rats (post hoc WT: Ftreatment(1,42)=3.89, p=0.03, veh vs 0.06 mg/kg: p=0.03; veh vs 0.2mg/kg: p=0.18; 0.06 vs 0.2 mg/kg: p=0.99; KO: Ftreatment(1,25)=7.71, p=0.002, veh vs 0.06 mg/kg: p=0.08; veh vs 0.2mg/kg: p=0.002; 0.06 vs 0.2 mg/kg: p=0.46, 3D). Consistent with the experiments without drug treatment (see Fig. 1), male nrxn1-KO rats played more than WT animals, showing increases in both pouncing (Fgenotype(1,67)=17.72, p<0.001, 3A) and pinning (Fgenotype(1,67)=12,73, p=0.001, 3B). Nrxn1-KO rats spent less time on social and non-social exploration (social: Fgenotype(1,67)=17.77, p<0.001, 3C; non-social: Fgenotype(1,67)=7.53, p=0.008, 3D).
Treatment with methylphenidate (0-0.3-1.0 mg/kg) suppressed pouncing (Ftreatment(2,67)=19.80, p<0.001, 3E) and pinning (Ftreatment(2,67)=16.53, p<0.001, 3F), social exploration was not affected (Ftreatment(2,67)=1.29, p=0.28; Fgenotype*treatment(2,67)=1.97, p=0.15, 3G) and non-social exploration was increased similarly in both genotypes (Fgenotype*treatment(2,67)=3.01, p=0.06: Ftreatment(2,67)=12.96, p<0.001; post-hoc: vehicle vs 0.3 mg/kg: p=0.4; vehicle vs 1.0 mg/kg: p<0.001; 0.3 vs 1.0 mg/kg: p=0.003, 3H). Again, male nrxn1-KO rats played more than WT rats (pouncing: Fgenotype(1,67)=38.98, p<0.001; pinning: Fgenotype(1,67)=27.50, p<0.001) while social exploratory behaviour was significantly reduced (Fgenotype(1,67)=45.10, p<0.001) and non-social exploration was unaffected (Fgenotype(1,67)=0.77, p=0.38). The reduction in play after methylphenidate treatment was comparable in the two genotypes for pouncing (Fgenotype*treatment(2,67)=1.16, p=0.32). An interaction effect of genotype and treatment was found for the frequency of pinning (Fgenotype*treatment(2,67)=3.25, p=0.04), methylphenidate suppressed pinning in both genotypes (post hoc tests WT: Ftreatment(2,43)=9.62, p<0.001, vehicle vs 0.3 mg/kg: p=0.04; vehicle vs 1.0 mg/kg: p<0.001; 0.3 vs 1.0 mg/kg: p=0.21, nrxn1-KO: Ftreatment(2,28)=7.02, p=0.004, vehicle vs 0.3 mg/kg: p=0.50; vehicle vs 1.0 mg/kg: p=0.003; 0.3 vs 1.0 mg/kg: p=0.09). After every dose, WT rats played less than nrxn1-KO rats treated with the same dose (vehicle WT vs nrxn1-KO: t(21)=-2.92, p=0.008; 0.3 mg/kg WT vs nrxn1-KO: t(23)=-3.59, p=0.005; 1.0 mg/kg WT vs nrxn1-KO: t(23)=-2.32, p=0.04) Fig. 3.
Effects of risperidone and methylphenidate on social play behaviour in male WT and nrxn1-KO rats. Risperidone (A-D): pouncing (A), pinning (B), social exploration (C) and non-social exploration (D) after treatment with vehicle (white bars) or risperidone 0.06 (light grey bars) or 0.2 mg/kg (dark grey bars). WT(0 mg/kg): n=14 pairs, WT(0.06 mg/kg): n=16 pairs, WT(0.2 mg/kg): n=15 pairs, nrxn1-KO(0 mg/kg): n=9 pairs, nrxn1-KO(0.06 mg/kg): n=9 pairs, nrxn1-KO(0.2 mg/kg): n=10 pairs. Methylphenidate (E-H): pouncing (E), pinning (F), social exploration (G) and non-social exploration (H) after treatment with vehicle (white bars), or methylphenidate 0.3 (light grey bars) or 1 mg/kg (dark grey bars). WT(0 mg/kg): n=14 pairs, WT(0.3 mg/kg): n=15 pairs, WT(1.0 mg/kg): n=15 pairs, nrxn1-KO(0 mg/kg): n=9 pairs, nrxn1-KO(0.3 mg/kg): n=10 pairs, nrxn1-KO(1.0 mg/kg): n=10 pairs. Data are displayed as mean + SEM. * or $p<0.05, **p<0.01, *** or $$$p<0.001, #p<0.08, *significant difference between WT and KO at the same dose. $significant difference within one genotype between vehicle and the indicated dose. #trend within one genotype between vehicle and indicated dose
Consequences of social play deprivation in nrxn1-KO and WT rats
To investigate whether social play deprivation would exacerbate the nrxn1-KO phenotype, rats were socially isolated from PND21-42, and social behaviour was tested during adolescence (at 7 weeks of age) or adulthood (11 weeks of age). On P42, the animals were resocialised for 4 days prior to testing. At 7 weeks of age, there were no differences between male and female rats, therefore data were pooled. nrxn1-KO rats showed more social play behaviour (Fig 4A- B, left graphs), but no modulating effect of play deprivation was observed (pouncing: Fgenotype(1,68)=36.09, p<0.001; Fhousing(1,68)=0.17, p=0.69; Fgenotype*housing(1,68)=1.90, p=0.17; pinning: Fgenotype(1,68)=9.34, p=0.003; Fhousing(1,68)=2.44, p=0.12; Fgenotype*housing(1,68)=0.51, p=0.48). Although the overall frequency was very low (1 out of 18 pairs), sexual mounting behaviour was displayed by socially housed, but not play-deprived nrxn1-KO rats (Fgenotype(1,68)=5.47, p=0.02; Fhousing(1,68)=3.95, p=0.05; Fgenotype*housing(1,68)=6.50, p=0.01, 4C, left graph). Post hoc analysis showed that compared to socially housed WT rats, socially housed nrxn1-KO rats displayed significantly more sexual mountings (Fig 4C, left panel of left graph). Social exploratory behaviour (Fig 4D, left graph) was significantly reduced in nrxn1-KO rats compared to WT rats. Play deprivation did not affect this behaviour (Fgenotype(1,68)=4.38, p=0.04; Fhousing(1,68)=0.41, p=0.52; Fgenotype*housing(1,68)= 0.005, p=0.94). Nrxn1-KO rats spent significantly less time on non-social exploratory behaviour (Fgenotype(1,68)= 6.55, p=0.01; Fhousing(1,68)= 0.08, p=0.77; Fgenotype*housing(1,68)= 0.39, p=0.54).
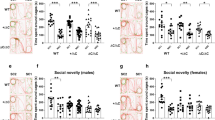
Effect of social play deprivation on social (play) behaviour in nrxn1-KO (KO) and wildtype (WT) rats, in adolescence (left set of graphs) and adulthood (right set of graphs). Pouncing (A), pinning (B), mounting (C), social exploration (D) and non-social exploration (E) in socially housed (left panels) nrxn1-KO (grey bars, black dots) compared to WT (white bars, black dots) rats, versus play-deprived (right panels) nrxn1-KO (grey bars, black squares) compared to WT rats (white grey bars, black squares). Data are displayed as means + SEM. #p=0.07, *p<0.05, **p<0.01, ***p<0.001. See Table 2 for all statistics including interactions. Adolescence (7 weeks, PND45-49): socially-housed WT rats: n=18 pairs, socially-housed nrxn1-KO rats: n=16 pairs, play-deprived WT rats: n=16 pairs, play-deprived nrxn1-KO rats: n=19 pairs; Adulthood (11 weeks, PND38-40) : socially-housed WT rats: n=10 pairs, socially-housed nrxn1-KO rats: n=9 pairs, play-deprived WT rats: n=12 pairs, play-deprived nrxn1-KO rats: n=12 pairs.
At 11 weeks of age (Fig. 4 A-E, right graphs, see Table 2 for all comparisons including statistics), adult nrxn1-KO animals played more than WT rats (Fig. 4A-B right panels). Social play deprivation did not affect play behaviour in adulthood, both pinning and pouncing were similar between housing conditions. Interestingly, whereas males and females played in equal amounts in adolescence, females made more pins in adulthood compared to males. In addition, female nrxn1-KO rats pinned more than either female WT and male nrxn1-KO rats (data not shown, see Table 2). Pouncing was similar between the sexes. nrxn-1 KO animals showed more boxing than WT animals but no effects of sex or housing condition were found. During social interactions, the amount of agonistic kicking was similar between the genotypes, sexes and housing conditions. Adult socially housed nrxn1-KO animals performed more sexual mounts compared to WT animals, while mounts in play-deprived animals were almost absent, and not different between genotypes (see Fig 4C, right graph). The number of mounts was not different between males and females.
Aspects of general social interest were recorded as well. Overall, play-deprived rats spent more time on social exploration compared those that were socially housed during PND21-42 (Fig 4D, right graph), but less on anogenital investigation (Table 2). These parameters were not influenced by genotype or sex. Nrxn1-KO rats spent more time following than WT rats, independent of housing, and females of both genotypes spent more time following than males. Nrxn-1 KO rats tended to spent less time on cage exploration compared to WT rats but this was independent of housing or sex (4E right graph).
Social interest in in nrxn1-KO and WT rats
Regardless of genotype or housing condition, the proportion of time spent investigating the unfamiliar animal compared to the empty cylinder was more than 50% in all groups (tWT/social(14)=9.62, p<0.001; tnrxn1-KO/social(9)=4.47, p=0.002; tWT/playdeprived(11)=4.04, p=0.002; tnrxn1-KO/playdeprived(11)=4.04, p=0.002), indicating a greater interest in a social stimulus compared to a non-social stimulus (Fig. 5A). The proportion of time spent on the social stimulus was not affected by nrxn1 deletion (Fgenotype(1,45)=0.41, p=0.53). However, socially housed animals spent a greater proportion of time close to the stimulus rat than play-deprived rats (Fhousing(1,45)=8.30, p=0.006, Fgenotype*housing(1,45)=0.31, p=0.58).
Effect of nrxn-1 KO and play deprivation on social interest and social discrimination in adult rats. A. Proportion of time spent in the vicinity of an unfamiliar stimulus rat versus an empty cylinder, in socially housed (social; left panels, black dots) and play deprived (right panels, black squares) WT (white bars) and nrxn1-KO (KO; grey bars) rats. B. Proportion of time spent in the vicinity of a novel unfamiliar rat versus the (now) familiar stimulus rat in socially housed (social; left panels, black dots) and play deprived (right panels, black squares) WT (white bars) and nrxn1-KO (KO; grey bars) rats. Data are presented as mean + SEM. *p<0.05, **p<0.01, ***p<0.001 versus 50%, ## p<0.01 social versus play deprived. WT social: n=15; nrxn1-KO social: n=10; WT play-deprived: n=12; nrxn1-KO play-deprived: n=12
In the next phase of the experiment (Fig. 5B), all groups spent slightly more than 50% of their time with the novel rat compared to the familiar one, but only significantly so in play-deprived nrxn1-KO rats (tnrxn1-KO/playdeprived(11)=5.13, p<0.001). The other groups did not significantly discriminate between the familiar and unfamiliar rat (tWT/social(14)=1.82, p=0.09; tnrxn1-KO/social(9)=0.92, p=0.38; tWT/playdeprived(11)=1.53, p=0.15, Fig 5B). No significant differences in proportion of time spent investigating the novel unfamiliar rat were found between the test groups (Fgenotype(1,45)=0.72, p=0.40; Fhousing(1,45)=1.53, p=0.22; Fgenotype*housing(1,45)=1.45, p=0.24).
Locomotor activity in adolescent and adult nrxn1-KO and WT rats
Locomotor activity was not affected by play deprivation, therefore data was pooled for this parameter. The rats were tested twice, during adolescence at 6 weeks (Fig. 6, left panel) and in adulthood at 11 weeks of age (Fig. 6, right panel). nrxn1-KO rats were more active than WT animals (adolescence: Fgenotype(1,90)=117.36, p<0.001; adulthood: Fgenotype(1,43)=66.91, p<0.001), and females were more active than males (adolescence: Fsex(1,90)=17.08, p<0.001; adulthood: Fsex(1,43)=15.21, p<0.001) and there was an interaction between genotype and sex (adolescence: Fgenotype*sex(1,90)=6.00, p=0.02; adulthood: Fgenotype*sex(1,43)=6.70, p=0.01). Post hoc testing revealed that in adolescence and adulthood both male and female NRXN1-KO rats were more active than WT rats. Furthermore, in adolescence and adulthood, female NRXN1-KO rats were more active than male KO rats . Female WT rats also showed more locomotor activity compared to male WT rats in adolescence but in adulthood, their locomotor activity was similar to that of males.
Locomotor activity in adolescent and adult nrxn1-KO and WT rats. Left panel: Locomotor activity in adolescent (6 weeks, PND38-40) male (M, black dots) and female (F, open dots) nrxn1-knock out (KO, grey bars) versus wildtype rats (WT, white bars). Right panel: locomotor activity of a subset of the same rats in adulthood (11 weeks, PND 75-77). Data are presented as mean + SEM. ***p<0.001 compared to its wildtype counterpart, $$p<0.01, males compared to females. Number of rats in adolescence: female WT rats: n=26, male WT rats: n=26, female nrxn1-KO rats: n=19, male nrxn1-KO rats: n=23; in adulthood: male WT rats: n=12, male nrxn1-KO rats: n=12, female WT rats: n=12, female nrxn1-KO rats: n=11
Locomotor response to amphetamine in nrxn1-KO and WT rats.
The effect of amphetamine on locomotor activity was investigated in adult males at 15 weeks of age. There was no difference between socially housed and play-deprived rats, therefore these data were pooled. Similar to the data in Fig. 6, during habituation to the test cage (Fig. 7A), nrxn1-KO rats were more active than WT rats (Fgenotype(1,121)=39.53, p<0.001; Ftime(1,121)=256.74, p<0.001, Ftime*genotype(1,121)=4.37, p=0.04).
Amphetamine-induced locomotor activity. Left panel: Habituation session of 30 minutes in male nrxn1-KO (KO; black line) versus wildtype (WT, grey line) rats. Data are presented as mean ± SEM per 10 minute time bin for WT (solid lines, n=61), and KO (dotted lines, n=62) rats. ***p<0.001. Right panel: Effect of amphetamine treatment in nrxn1-KO (KO; triangles) and WT (squares) rats. Data are presented as mean ± SEM, per 10 minute time bin for vehicle (light grey lines), 0.25 mg/kg (dark grey lines), and 0.5 mg/kg amphetamine treatment (black lines). WT vehicle: n=20, WT 0.25 mg/kg: n=21, WT 0.5 mg/kg: n=20, nrxn1-KO vehicle: n=18, nrxn1-KO 0.25 mg/kg: n=23, nrxn1-KO 0.5 mg/kg: n=21
After treatment with amphetamine, locomotor activity increased in both genotypes (Fdose(2,117)=66.96, p<0.001), whereby activity was higher in the nrxn1-KO rats (Fgenotype*dose(2,117)=3.17, p=0.046). Post hoc analysis revealed that locomotor activity was higher in nrxn1-KO rats compared to WT rats after treatment with vehicle (t(38)=-2.86, p=0.007) and the 0.5 mg/kg dose of amphetamine (t(39)=-3.43, p=0.001), but not the 0.25 mg/kg dose of amphetamine (t(42)=-1.54, p=0.13).
Discussion
Proper social functioning, important throughout life, is impaired in neurodevelopmental disorders of which disruptions in the genes coding for the transsynaptic communication protein neurexin-1 is a risk factor. The purpose of this study was to assess the impact of nrxn1 deletion on the developmental trajectory of social (play) behaviour in rats, from the juvenile phase until adulthood. Furthermore, on the basis of the idea that psychiatric symptomatology is precipitated by life events in genetically predisposed individuals, we studied whether actively depriving the rats of play during their juvenile period would affect their behaviour later in life depending on the presence of this genetic vulnerability. We also investigated the effects of drugs commonly used in clinical practice to alleviate specific symptoms common to these diseases.
The main findings of these studies were that nrxn1-KO rats displayed strongly exaggerated social play behaviour throughout development compared to littermate controls, and were more motivated to engage in play. Moreover, age-inappropriate sexual mounting behaviour was observed in the nrxn1-KO rats. Nrxn1-KO rats also showed increased locomotor activity. Play deprivation subtly affected social behaviour in adulthood, but did not profoundly influence the nrxn1-KO phenotype. Treatment with risperidone and methylphenidate inhibited social play to a comparable extent in WT and nrxn1-KO rats, and an exaggerated locomotor response to amphetamine was observed in nrxn1-KO rats.
The social behavioural phenotype of neurexin-1 KO rats
Nrxn1-KO rats showed higher levels of social play behaviour compared to their WT counterparts from their juvenile age throughout young adulthood. This robust effect was consistently observed in different cohorts of rats, in males as well as females. Both the initiation to play, pouncing, as well as the number of pins were higher in juvenile (4 weeks), and adolescent (7 weeks) nrxn1-KO rats, while social exploratory behaviour was reduced. In addition to the high levels of actual play behaviour, the motivation for social play behaviour was also higher in juvenile nrxn1-KO rats. The nrxn1-KO rats remained more playful until -at least- 11 weeks of age. In adulthood (7 months), similar levels of social approach behaviour as well as intact social discrimination were seen in the three-chamber task.
The majority of KO rats exhibited inappropriate sexual mounting behaviour towards same-sex and same-genotype social interaction partners (see also Twining et al. 2017), which was virtually absent in WT animals. This mounting behaviour started as early as week 4 of age and was still present in adulthood at 11 weeks of age. The neurobehavioural underpinnings of this aberrant behaviour remain to be identified, but it is interesting to note that inappropriate social and sexual behaviour also feature in ASD. Although sexual behaviour is an understudied aspect of ASD, in a recent review, a higher prevalence of altered sexual function was reported (Maggio et al. 2022).
The strong increase in play behaviour in the nrxn1-KO rats in our study is in contrast to the decrease in play found previously by Kight et al. (2021). Although that study used rats of the same age, and also studied same-genotype unfamiliar partners, there are also important procedural differences, which may explain the discrepancy in the obtained results. That is, the Kight et al. (2021) study scored behaviour of individual rats, averaged over 4 days of testing in a non-habituated setting. In contrast, here we looked at the play behaviour of a pair of juveniles, that were habituated to the setup on the days preceding the test. Moreover, in the present study, we isolated the animals for 2.5 hours before testing, whereas no isolation was used by Kight et al.. This isolation period is routinely used in our laboratory to evoke reliable, half-maximal levels of social play (Niesink and Van Ree 1989; Vanderschuren et al. 1995; 2008), resulting in five- to tenfold higher levels of play than in the Kight et al. study. It may therefore be that nrxn1-KO rats are more aroused by the encounter with a conspecific after the isolation. In addition, the profound hyperactivity of the nrxn1-KO rats in a novel environment (Esclassan et al. 2015; Kight et al. 2021; present study) may have interfered with the expression of social play in the Kight study, while habituation to the test environment in the present study will have mitigated this interference.
In addition to the increase in social play behaviour, the motivation for social play behaviour was also higher in the nrxn1-KO rats. The rewarding properties of social play behaviour in rats have been well-documented in place conditioning and operant conditioning setups (Calcagnetti and Schechter 1992; Trezza et al. 2011; Achterberg et al. 2016a,b, 2019). Together with the profound and consistent increase in social play observed in the nrxn1-KO rats, the heightened levels of responding for social play suggest that the rewarding value of play is enhanced in the nrxn1-KO rats. It is well-known that incentive motivation for rewards depends on dopaminergic neurotransmission in the nucleus accumbens (Salamone et al. 2012). Indeed, dopamine has been implicated in the motivation for social play (Achterberg et al. 2015) and directly stimulating dopaminergic signalling in the nucleus accumbens enhances social play behaviour (Manduca et al. 2016). Whether there is a heightened motivation for rewards in general in the nrxn1-KO rats or whether this is specific for play, remains to be studied. In any event, our observations are not consistent with a phenotype of reduced social reward, as has been suggested for ASD (Chevallier et al. 2012). The increase in social play behaviour in nrxn1-KO rats was not observed in the reinforced trials of the operant paradigm. This is because the operant conditioning setup, animals have only one minute to play per reinforced period, whereas social play expression is analysed for 15 minutes continuously. It could be that because the playful interaction is interrupted after one minute, the stimulating effects on social play are less likely to arise. The present data, together with our previous findings (Achterberg et al. 2016a,b; 2019) therefore suggest that social play expression in our operant setup may be more sensitive to manipulations that decrease social play than to those that increase this behaviour.
Alongside the increases in social play behaviour, we also observed marked locomotor hyperactivity in the nrxn1-KO rats, consistent with previous studies (Esclassan et al. 2015; Kight et al. 2021). We think that it is unlikely, however, that the enhanced social play results from mere hyperactivity, for several reasons. Thus, while play was enhanced, social exploratory behaviour was reduced, arguing against a general increase in social behaviours as a result of hyperactivity. In addition, non-social exploratory behaviour in the social test setting was not altered in the nrxn1-KO rats. Furthermore, locomotor hyperactivity and social play behaviour can be pharmacologically dissociated. For example, treatment with psychostimulant drugs in doses that evoke hyperactivity, typically results in a reduction in social play (for reviews see Vanderschuren et al. 2016; Achterberg and Vanderschuren 2023), and we have previously shown that treatment with methylphenidate in a social setting does not alter locomotion, yet suppresses social play (Vanderschuren et al. 2008). In fact, one could also argue (see above) that profound hyperactivity compromises the cognitive and motoric mechanisms necessary for the proper expression of social play. Therefore, we interpret our findings as that deletion of the nrxn1 gene results in a (socially) disinhibited phenotype, rather than that the hyperactivity of the nrxn1-KO rats non-specifically enhances any behaviour likely to occur in a given setting. This lack of inhibition in both social and non-social situations has been reported in children diagnosed with ASD (Davidson et al. 2015; Mayes et al. 2017).
Modest behavioural consequences of social play deprivation
In the present study, we introduced deprivation of juvenile social play experience, i.e. social isolation during the three weeks in life when social play is most abundant, as a negative life event. We hypothesized that deprivation of social play would act as a second ‘hit’ on top of the genetic vulnerability conferred by the nrxn1 deletion to precipitate or exacerbate ASD- or SCZ-like aberrant behaviours. Indeed, impaired social interactions or social withdrawal during youth is thought to worsen the symptoms of ASD and SCZ (Helgeland and Torgersen 2005; Jones et al. 1994; Jordan 2003), and previous studies have shown that deprivation of social play leads to long-lasting impairments in the social, emotional and cognitive domain (Baarendse et al. 2013; Bijlsma et al. 2022; Lukkes et al. 2009a; Potegal and Einon 1989; Van den Berg et al. 1999a; Whitaker et al. 2013, for reviews see Vanderschuren and Trezza 2014; Pellis et al. 2023). Contrary to our hypothesis, however, we found no robust additional or modifying effects of play deprivation on social (play) behaviour that differed between genotypes, with one exception. That is, social play deprivation almost abolished the sexual mounting in the nrxn1-KO rats, suggesting that in this case, one aberration counteracted another one. The underlying mechanism of this fascinating effect remains to be identified, but we speculate that the reorganization of the social repertoire as a result of play deprivation (whereby animals have particular difficulties coping with challenging social situations, see below) may not allow for this inappropriate behaviour to be displayed. Social play deprivation also resulted in modest increases in social exploratory behaviour and reduced anogenital investigation in adult rats, thus slightly altering the way in which conspecifics are investigated. In the 3-chamber social approach task, play-deprived animals were attracted by a social stimulus but slightly less so than socially-housed animals. These findings on social behaviour are somewhat consistent with previous studies, that reported modest reductions in social exploratory behaviour after play deprivation (Hol et al. 1999; Van den Berg et al. 1999a, b, c; Lukkes et al. 2009a,b). Whereas in the present study we focused on the more appetitive aspects of social behaviour, previous studies have shown that in challenging social situations, such as in an encounter with an aggressive animal, play-deprived animals are less able to use the appropriate social signals to manage conflict (Van den Berg et al. 1999a, b, c; Von Frijtag et al. 2002). It may therefore be that for an interaction between play deprivation and nrxn1 deletion to become apparent, socially challenging encounters need to be assessed, rather than the relatively safe social settings used here. Taken together, play deprivation resulted in subtle social alterations but did not act synergistically to produce a specific or more severe social phenotype. Whether play deprivation leads to enhanced cognitive deficits or alterations in emotional reactivity on top of the genetic vulnerability will be the focus of future research.
Risperidone and methylphenidate do not selectively affect play behaviour in nrxn1-KO rats, but nrxn1-KO rats are more sensitive to amphetamine
In order to put the exaggerated social play behaviour in nrxn1-KO rats into a clinical perspective, and to gauge the underlying neuropharmacological mechanisms, especially the monoamine neurotransmitter systems, we investigated the effects of risperidone and methylphenidate on social play behaviour. Risperidone is a dopamine D2/serotonin 5-HT2 receptor antagonist, that is used to treat irritability and aggression in ASD and SCZ (McCracken et al. 2002; Shea et al. 2004), and methylphenidate is a dopamine and noradrenaline reuptake inhibitor, that is used for the treatment of inattention, hyperactivity and impulsivity, that are typical of attention-deficit hyperactivity disorder (ADHD; Biederman and Faraone 2005; Feldman and Reiff 2014), but that also occurs in ASD (Murray 2010; Krakowski et al. 2020). In addition, both risperidone and methylphenidate have been shown to alleviate social deficits in SCZ and ASD animal models as well as patients (Hara et al. 2016; Kamińska and Rogóż, 2015; Wang et al. 2007; Smith et al. 1998; Harvey et al. 2006). To investigate the sensitivity to psychostimulant drugs, we assessed the hyperactivity evoked by amphetamine (that stimulates the release of dopamine, noradrenaline and serotonin), that is also used for the treatment of ADHD (Biederman and Faraone 2005; Feldman and Reiff 2014) but exacerbates positive symptoms of SCZ (Meltzer and Stahl 1976; Kapur 2003).
Risperidone reduced social play behaviour to an equal extent in both genotypes. This antipsychotic is thought to exert its effects through antagonism of dopamine D2 and serotonin 5-HT2 receptors, but it also antagonizes noradrenergic alpha-1 and -2 and histaminergic H1 receptors. Treatment with dopamine D2 and alpha-1 receptor antagonists has previously been shown to inhibit social play, whereas treatment with an alpha-2 adrenoceptor antagonist actually enhances it (Siviy et al. 1994; -1996). The effects of serotonin 5-HT2 and histamine H1 receptor antagonism on social play remain to be properly investigated. Thus, the play-suppressant effect of risperidone likely depends on blockade of dopamine D2 receptors, alpha-1 adrenoceptors, or both, whereby the activity of these receptor systems does not differ between WT and nrxn1-KO rats. That said, the possibility that risperidone suppresses social play behaviour through distinct pharmacological mechanisms in the two genotypes can as yet not be excluded. From a clinical point of view, the comparable sensitivity to risperidone in WT and nrxn1-KO rats argues against the possibility that the increased play behaviour in nrxn1-KO rats reflects heightened levels of aggression, which is suggested by the increased levels of boxing, kicking and chasing observed in young adult nrxn1-KO rats – an explanation that would also be unlikely given the structural differences in social play and aggression in rats (e.g. Pellis and Pellis 1987). Moreover, the fact that risperidone did not affect social exploration and increases non-social exploration indicates that at the doses tested, the reduction in social play cannot be explained by general sedative effect of D2 receptor blockade.
Methylphenidate, the most prescribed drug for the treatment of ADHD, reduced social play behaviour in both genotypes without affecting social and non-social exploration. This specific reduction in social play after methylphenidate treatment is consistent with previous findings (Beatty et al. 1982; Vanderschuren et al. 2008), and we have demonstrated that methylphenidate inhibits social play behaviour through indirect stimulation of alpha-2 adrenoceptors (Vanderschuren et al. 2008; Achterberg et al. 2016a, b). The comparable sensitivity to methylphenidate in WT and nrxn1-KO rats indicates that the increase in social play is not the result of altered alpha-2 noradrenergic signalling in nrxn1-KO rats. We have previously argued that the play-suppressant properties of methylphenidate are due to increased behavioural inhibition, whereby energetic behaviours that result in diminished attention for the environment are reduced (Vanderschuren et al. 2008). That said, the subsequent findings that methylphenidate reduces social play through both prefrontal and subcortical limbic structures implies that alongside increased – prefrontal – inhibition, methylphenidate may also alter the – subcortically mediated – emotional properties of social play (Achterberg et al. 2015). A disinhibited phenotype could therefore underlie the exaggerated social play in nrxn1-KO rats, but it is not one that results from altered noradrenergic neurotransmission.
We also assessed the sensitivity to the psychomotor effects of amphetamine. In this experiment, nrxn1-KO rats were hyperactive, as was found previously (Esclassan et al. 2015; Kight et al. 2021), and although amphetamine-induced hyperactivity was observed in both WT and nrxn1-KO animals, the latter showed a heightened response to the highest dose of amphetamine tested. Given that both novelty-induced locomotor activity (Hooks and Kalivas 1994) and the hyperactivity evoked by amphetamine depend on nucleus accumbens dopamine neurotransmission (Creese and Iversen 1975; Pijnenburg et al. 1975), these findings suggest that nucleus accumbens dopaminergic neurotransmission is hypersensitive in nrxn1-KO rats. Increased sensitivity of mesolimbic dopamine has been implicated in SCZ (Kapur 2003), and it has also been observed in the methylazoxymethanol acetate model of SCZ (e.g. Gomes et al. 2014). Thus, the increased sensitivity to amphetamine in nrxn1-KO rats is consistent with an SCZ-like phenotype. Conversely, the lack of a corrective effect of amphetamine treatment on the nrxn1-KO phenotype in the direction of WT suggests that this overactive locomotor phenotype might not be ADHD-like hyperactivity.
Conclusion
In the present study, we demonstrate an aberrant social phenotype of nrxn1-KO rats, apparent as exaggerated social play, increased motivation for play and inappropriate sexual mounting. In contrast to our hypothesis, nrxn1-KO rats did not display reduced social interaction or social withdrawal reminiscent of ASD or SCZ symptomatology, and the intended ‘second hit’ of social play deprivation had no major consequences for social behaviour. Nrxn1-KO rats displayed an overall increase in general activity, increased responsiveness to amphetamine, but risperidone and methylphenidate did not selectively alter the social phenotype of nrxn1-KO rats. Together, these findings suggest that deletion of the nrxn1 gene results in a disinhibited phenotype, in both a social and a non-social setting. The neurexin-1 knockout rat could therefore be used as a model for inappropriate or disinhibited social behaviour seen in childhood mental disorders.
References
Achterberg EJM, Vanderschuren LJMJ (2023) The neurobiology of social play behaviour: past, present and future. Neurosci Biobehav Rev 152:105319
Achterberg EJM, Trezza V, Siviy SM, Schrama L, Schoffelmeer AN, Vanderschuren LJMJ (2014) Amphetamine and cocaine suppress social play behavior in rats through distinct mechanisms. Psychopharmacology 231:1503–1515
Achterberg EJM, Van Kerkhof LWM, Damsteegt R, Trezza V, Vanderschuren LJMJ (2015) Methylphenidate and atomoxetine inhibit social play behavior through prefrontal and subcortical limbic mechanisms in rats. J Neurosci 35:161–169
Achterberg EJM, Van Kerkhof LWM, Servadio M, Van Swieten MMH, Houwing DJ, Aalderink M, Driel NV, Trezza V, Vanderschuren LJMJ (2016) Contrasting roles of dopamine and noradrenaline in the motivational properties of social play behavior in rats. Neuropsychopharm 41:858–868
Achterberg EJM, van Swieten MMH, Driel NV, Trezza V, Vanderschuren LJMJ (2016) Dissociating the role of endocannabinoids in the pleasurable and motivational properties of social play behaviour in rats. Pharm Res 110:151–158. https://doi.org/10.1016/j.phrs.2016.04.031
Achterberg EJM, Damsteegt R, Vanderschuren LJMJ (2018) On the central noradrenergic mechanism underlying the social play-suppressant effect of methylphenidate in rats. Behav Brain Res 347:158–166
Achterberg, EJM, van Swieten, MMH, Houwing, DJ, Trezza, V, Vanderschuren, LJMJ. (2019). Opioid modulation of social play reward in juvenile rats. Neuropharm. 159:[107332]. https://doi.org/10.1016/j.neuropharm.2018.09.007
Argue KJ, McCarthy MM (2015) Characterization of juvenile play in rats: Importance of sex of self and sex of partner. Biology of Sex Diff 6(1):16
Armstrong EC, Caruso A, Servadio M, Andreae LC, Trezza V, Scattoni ML, Fernandes C (2020) Assessing the developmental trajectory of mouse models of neurodevelopmental disorders: Social and communication deficits in mice with Neurexin 1α deletion. Genes Brain Behav. 19(4):e12630. https://doi.org/10.1111/gbb.12630
Baarendse PJJ, Counotte DS, O’Donnell P, Vanderschuren LJMJ (2013) Early social experience is critical for the development of cognitive control and dopamine modulation of prefrontal cortex function. Neuropsychopharmacol 38:1485–1494
Baarendse PJJ, Limpens JHW, Vanderschuren LJMJ (2014) Disrupted social development enhances the motivation for cocaine in rats. Psychopharmacology 231(8):1695–1704
Baenninger LP (1967) Comparison of behavioural development in socially isolated and grouped rats. Animal Behaviour 15(2–3):312–323
Beatty WW, Dodge AM, Dodge LJ, Panksepp J (1982) Psychomotor stimulants, social deprivation and play in juvenile rats Pharmacol Biochem Behav 16:417–422
Biederman J, Faraone SV (2005) Attention-deficit hyperactivity disorder. Lancet 366:237–248
Bijlsma A, Omrani A, Spoelder M, Verharen JPH, Bauer L, Cornelis C, de Zwart B, van Dorland R, Vanderschuren LJMJ, Wierenga CJ (2022) Social play behavior is critical for the development of prefrontal inhibitory synapses and cognitive flexibility in rats. J Neurosci 42:8716–8728
Blundell J, Blaiss CA, Etherton MR, Espinosa F, Tabuchi K, Walz C, Powell CM (2010) Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci 30(6):2115–2129
Calcagnetti DJ, Schechter MD (1992) Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol Behav 51:667–672
Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT (2012) The social motivation theory of autism. Trends Cogn Sci 16:231–239
Ching MSL, Shen Y, Tan W-H, Jeste SS, Morrow EM, Chen X, Mukaddes NM, Yoo S-Y, Hanson E, Hundley R, Austin C, Becker RE, Berry GT, Driscoll K, Engle EC, Friedman S, Gusella JF, Hisama FM, Irons MB, Lafiosca T, LeClair E, Miller DT, Neessen M, Picker JD, Rappaport L, Rooney CM, Sarco DP, Stoler JM, Walsh CA, Wolff RR, Zhang T, Nasir RH, Wu B-L (2010) Deletions of NRXN1 (neurexin-1) predispose to a wide spectrum of developmental disorders. Am J Med Genet B 153:937–947
Creese I, Iversen SD (1975) The pharmacological and anatomical substrates of the amphetamine response in the rat. Brain Res 83:419–436
Dachtler J, Ivorra JL, Rowland TE, Lever C, Rodgers RJ, Clapcote SJ (2015) Heterozygous deletion of α-neurexin I or α-neurexin II results in behaviors relevant to autism and schizophrenia. Behav Neurosci 129(6):765–76. https://doi.org/10.1037/bne0000108
Davidson C, O’Hare A, Mactaggart F, Green J, Young D, Gillberg C, Minnis H (2015) Social relationship difficulties in autism and reactive attachment disorder: Improving diagnostic validity through structured assessment. Res Dev Disabil 40:63–72. https://doi.org/10.1016/jridd201501007
Douglas LA, Varlinskaya EI, Spear LP (2004) Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Developmental Psychobiology 45(3):153–162
Einon DF, Morgan MJ (1977) A critical period for social isolation in the rat. Dev Psychobiol 10:123–132
Einon DF, Morgan MJ, Kibbler CC (1978) Brief periods of socialization and later behavior in the rat. Develop Psychobiology 11(3):213–225
Esclassan F, Francois J, Phillips KG, Loomis S, Gilmour G (2015) Phenotypic characterization of nonsocial behavioral impairment in neurexin 1α knockout rats. Behav Neurosci 129(1):74–85
Etherton MR, Blaiss CA, Powell CM, Südhof TC (2009) Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci U S A 106(42):17998–8003. https://doi.org/10.1073/pnas.0910297106
Feldman HM, Reiff MI (2014) Attention deficit–hyperactivity disorder in children and adolescents. N Engl J Med 370:838–846
Gauthier J, Siddiqui TJ, Huashan P, (…), Craig, AM, Rouleau, GA, (2011) Truncating mutations in NRXN2 and NRXN1 in autism spectrum disorders and schizophrenia. Human Gen 130(4):563–573
Gejman PV, Sanders AR, Duan J (2010) The role of genetics in the etiology of schizophrenia. Psychiatr Clin North Am 33:35–66
Glessner JT, Wang K, Cai G, Korvatska O, Kim CE, Schellenberg GD, Hakonarson H (2009) Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature 459:569–573
Gomes FV, Guimarães FS, Grace AA (2014). Effects of pubertal cannabinoid administration on attentional set-shifting and dopaminergic hyper-responsivity in a developmental disruption model of schizophrenia. Int J Neuropsychopharmacol. 13;18(2):pyu018. 101093/ijnp/pyu018
Graham KL, Burghardt GM (2010) Current perspectives on the biological study of play: signs of progress. Q Rev. Biol. 85:393–418
Hamilton SM, Green JR, Veeraragavan S, Weinstein E, Paylor R (2014) Fmr1 and Nlgn3 knockout rats: Novel tools for investigating autism spectrum disorders. Behav Neurosci 128(2):103–109
Hara Y, Ago Y, Taruta A, Katashiba K, Hasebe S, Takano, E, ..., Takuma K (2016). Improvement by methylphenidate and atomoxetine of social interaction deficits and recognition memory impairment in a mouse model of valproic acid‐induced autism. Autism Research, 9(9) 926-939
Harvey PD, Patterson TL, Potter IS et al (2006) Improvement in social competence with short-term atypical antipsychotic treatment: A randomized, double-blind comparison of quetiapine versus risperidone for social competence, social cognition, and neuropsychological functioning. Am J Psychiatry 163:1918–1925
Helgeland MI, Torgersen S (2005) Stability and prediction of schizophrenia from adolescence to adulthood. Eur Child Adolescent Psych 14(2):83–94
Hodos W (1961) Progressive ratio as a measure of reward strength. Science 134:934–944
Hol T, Van den Berg CL, Van Ree JM, Spruijt BM (1999) Isolation during the play period in infancy decreases adult social interactions in rats. Behav Brain Res 100:91–97
Hooks MS, Kalivas PW (1994) Involvement of dopamine and excitatory amino acid transmission in novelty-induced motor activity. J Pharmacol Exp Ther 269:976–988
Imamura A, Morimoto Y, Ono S, Kurotaki N, Kanegae S, Yamamoto N, Kinoshita H, Tsujita T, Okazaki Y, Ozawa H (2020) Genetic and environmental factors of schizophrenia and autism spectrum disorder: insights from twin studies. J Neural Transm 127:1501–1515
Janz P, Bainier M, Marashli S, Schoenenberger P, Valencia M, Redondo RL (2022) Neurexin1α knockout rats display oscillatory abnormalities and sensory processing deficits back-translating key endophenotypes of psychiatric disorders. Transl Psych 12:455. https://doi.org/10.1038/s41398-022-02224-1
Jarrold C (2003) A review of research into pretend play in autism. Autism 7:379–390
Jones P, Murray R, Rodgers B, Marmot M (1994) Child developmental risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet 344(8934):1398–1402
Jordan R (2003) Social play and autistic spectrum disorders: A perspective on theory, implications and educational approaches. Autism 7(4):347–360
Kamińska K, Rogóż Z (2015) The effect of combined treatment with risperidone and antidepressants on the MK-801-induced deficits in the social interaction test in rats. Pharmacological Rep 67(6):1183–1187
Kapur S (2003) Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psych 160:13–23
Kentrop, J, Smid, CR, Achterberg, EJM, van IJzendoorn, MH, Bakermans-Kranenburg, MJ, Joels, M, van der Veen, R, (2018). Effects of maternal deprivation and complex housing on rat social behavior in adolescence and adulthood. Front. Behav. Neurosci. 12:193
Kight KE, Argue KJ, Bumgardner JG, Bardhi K, Waddell J, McCarthy MM (2021) Social behavior in prepubertal neurexin 1α deficient rats: A model of neurodevelopmental disorders. Behav Neurosci 135(6):782–803
Kim HG, Kishikawa S, Higgins AW, Seong IS, Morton CC, Quade BJ, Gusella JF (2008) Disruption of neurexin 1 associated with autism spectrum disorder Am J Hum Genet 82:199-207
Krakowski AD, Cost KT, Anagnostou E, Lai MC, Crosbie J, Schachar R et al (2020) Inattention and hyperactive/impulsive component scores do not differentiate between autism spectrum disorder and attention-deficit/hyperactivity disorder in a clinical sample. Mol Autism 11(1):1–13
Ku KM, Weir RK, Silverman JL, Berman RF, Bauman MD (2016) Behavioral phenotyping of juvenile Long-Evans and Sprague-Dawley rats: Implications for preclinical models of autism spectrum disorders. PLoS ONE 11(6):e0158150
Lamy M, Pedapati EV, Dominick KL, Wink LK, Erickson CA (2020) Recent Advances in the Pharmacological Management of Behavioral Disturbances Associated with Autism Spectrum Disorder in Children and Adolescents. Pedia Drugs 22:473–483
Lesscher, HMB, Spoelder, M, Rotte, MD, Baars, AM, Vanderschuren, LJMJ (2015). Early social isolation augments alcohol consumption in rats. Behavioural Pharmacology 26:673-680
Leussis MP, Andersen SL (2008) Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse 62(1):22–30
Lukkes JL, Vuong S, Scholl JL, Olivier H, Forster GL (2009) Corticotropin-releasing factor receptor antagonism within the dorsal raphe nucleus reduces social anxiety-like behavior after early life social isolation. J Neurosci 29:9955–9960
Lukkes JL, Mokin MV, Scholl JL, Forster GL (2009) Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm Behav 55:248–256
Maggio MG, Calatozzo P, Cerasa A, Pioggia G, Quartarone A, Calabrò RS (2022) Sex and sexuality in autism spectrum disorders: a scoping review on a neglected but fundamental issue. Brain Sci 12:1427
Manduca A, Servadio M, Damsteegt R, Campolongo P, Vanderschuren LJMJ, Trezza V (2016) Dopaminergic neurotransmission in the nucleus accumbens modulates social play behavior in rats. Neuropsychopharmacol 41:2215–2223
Manning MM, Wainwright LD (2010) The role of high level play as a predictor social functioning in autism. J Autism Dev Disord 40:523–533
Marder SR, Galderisi S (2017) The current conceptualization of negative symptoms in schizophrenia. World Psychiatry 16:14–24
Mayes SD, Calhoun SL, Waschbusch DA, Baweja R (2017) Autism and reactive attachment/disinhibited social engagement disorders: Co-occurrence and differentiation. Clin Child Psychology Psych 22(4):620–631. https://doi.org/10.1177/1359104516678039
McCracken JT, McGough J, Shah B et al (2002) Risperidone in children with autism and serious behavioral problems. N Engl J Med 347(5):314–321
McCutcheon JE, Marinelli M (2009) Age matters. Eur J Neurosci 29:997–1014
Meaney MJ, Stewart J (1981) A descriptive study of social development in the rat (Rattus norvegicus). Animal Behaviour 29:34–45
Meltzer HY, Stahl SM (1976) The dopamine hypothesis of schizophrenia: a review. Schizophr Bull 2:19–76
Meng Q, Li N, Han X, Shao F, Wang W (2010) Peri-adolescence isolation rearing alters social behavior and nociception in rats. Neurosci Lett 480:25–29
Modi, ME, Brooks, JM, Guilmette, ER, O’Donnell, P, Buhl, DL (2018). Hyperactivity and hypermotivation associated with increased striatal mglur1 signalling in a Shank2 rat model of autism. Front Molecular Neurosci 11:107
Møller R, Husby R (2000) The Initial Prodrome in Schizophrenia: Searching for Naturalistic Core Dimensions of Experience and Behavior. Schizophr Bull 26(1):217–232
Murray MJ (2010). Attention-deficit/Hyperactivity Disorder in the Context of Autism Spectrum Disorders. Curr Psychiatry Rep 12, 382–388. https://doi.org/10.1007/s11920-010-0145-3
Nickels KC, Katusic SK, Colligan RC, Voigt RG, Barbaresi WJ (2008) Stimulant medication treatment of target behaviors in children with autism: A population-based study. J Develop Behav Pedia 29(2):75–81
Niesink RJ, Van Ree JM (1982) Short-term isolation increases social interactions of male rats: a parametric analysis. Physiol Behav 29(5):819–825
Niesink RJM, Van Ree JM (1989) Involvement of opioid and dopaminergic systems in isolation- induced pinning and social grooming of young rats. Neuropharmacol 28:411–418
Panksepp J (1981) The ontogeny of play in rats. Dev Psychobiol 14:327–332
Panksepp J, Beatty WW (1980) Social deprivation and play in rats. Behav Neural Biol 30:197–206
Panksepp J, Siviy SM, Normansell L (1984) The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev 8:465–492
Pellis SM, Pellis VC (2009) The Playful Brain. OneWorld Publications, Oxford, UK
Pellis SM, Pellis VC, Ham JR, Stark RA (2023) Play fighting and the development of the social brain: The rat’s tale. Neurosci Biobehav Rev 145:105037
Pellis SM, Pellis VC (1987). Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat Rattus norvegicus Aggr Behav 13:227-242
Pijnenburg AJJ, Honig WMM, Van Rossum JM (1975) Inhibition of d-amphetamine-induced locomotor activity by injection of haloperidol into the nucleus accumbens of the rat. Psychopharmacol 41:87–95
Poole TB, Fish J (1975) An investigation of playful behavior in Rattus norvegicus and Mus musculus (Mammalia). J Zool Lond 175:61–71
Potegal M, Einon DF (1989) Aggressive behaviors in adult rats deprived of play fighting experienced as juveniles. Dev Psychobiol 22:159–172
Rabaneda LG, Robles-Lanuza E, Nieto-González JL, Scholl FG (2014) Neurexin dysfunction in adult neurons results in autistic-like behavior in mice. Cell Rep 24;8(2):338-346. https://doi.org/10.1016/j.celrep.2014.06.022
Richardson NR, Roberts DC (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Meth 66:1–11
Rujescu D, Ingason A, Cichon S, St Clair D, Stefansson H, Collier DA (2009) Disruption of the neurexin 1 gene is associated with schizophrenia. Hum Mol Genet 18:988–996
Sahoo T, Theisen A, Rosenfeld JA, Bejjani BA, Shaffer LG (2011) Copy number variants of schizophrenia susceptibility loci are associated with a spectrum of speech and developmental delays and behavior problems. Gen Med 13(10):868–880
Salamone JD, Correa M (2012) The mysterious motivational functions of mesolimbic dopamine. Neuron 76:470–485
Scott KE, Schormans AL, Pacoli KY, Allman BL, Schmid S (2018) Altered auditory processing, filtering, and reactivity in the cntnap2 knock-out rat model for neurodevelopmental disorders. J Neurosci 38(40):8588–8604
Scott KE, Kazazian K, Mann RS, Schmid S, Allman BL (2020) Loss of Cntnap2 in the Rat Causes Autism-Related Alterations in Social Interactions, Stereotypic Behavior, and Sensory Processing. Autism Res 13(10):1698–1717
Shea S, Turgay A, Carroll A et al (2004) Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pedia 114(5):e634-641
Shields H (1980) Genetics and mental development. In: Child Psychiatry: Modern Approaches, Rutter, M. (ed). Oxford: Blackwell Scientific
Siviy SM, Panksepp J (1987) Sensory modulation of juvenile play in rats Developmental Psychobiology. J Inter Soc Develop Psychobio 20(1):39–55
Siviy SM, Fleischhauer AE, Kuhlman SJ, Atrens DM (1994) Effects of alpha-2 adrenoceptor antagonists on rough-and-tumble play in juvenile rats: evidence for a site of action independent of non-adrenoceptor imidazoline binding sites. Psychopharmacol 113:493–499
Siviy SM, Fleischhauer AE, Kerrigan LA, Kuhlman SJ (1996) D2 dopamine receptor involvement in the rough-and-tumble play behavior of juvenile rats. Behav Neurosci 110:1168–1176
Smith BH, Pelham WE, Evans S, Gnagy E, Molina B, Bukstein O, ... Willoughby M (1998). Dosage effects of methylphenidate on the social behavior of adolescents diagnosed with attention deficit hyperactivity disorder. Exp.erimental and Clin.ical Psychopharmacology, 6(2) 187
Spear LP (2000) The adolescent brain and age related behavioral manifestations. Neurosci Biobehav Rev 24(4):417–463
Spinka M, Newberry RC, Bekoff M (2001) Mammalian play: training for the unexpected. Q Rev Biol 76:141–168
Südhof TC (2008) Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455:903–911
Thomas AM, Schwartz MD, Saxe MD, Kilduff TS (2017) Sleep/wake physiology and quantitative electroencephalogram analysis of the neuroligin-3 knockout rat model of autism spectrum disorder. Sleep 40(10):138
Tóth M, Halász J, Mikics E, Barsy B, Haller J (2008) Early Social Deprivation Induces Disturbed Social Communication and Violent Aggression in Adulthood. Behav Neurosci 122(4):849–854
Trezza V, Baarendse PJJ, Vanderschuren LJMJ (2010) The pleasures of play: Pharmacological insights into social reward mechanisms. Trends Pharmacol Sci 31(10):463–469
Trezza V, Campolongo P, Vanderschuren LJMJ (2011) Evaluating the rewarding nature of social interactions in laboratory animals. Dev Cogn Neurosci 1:444–457
Twining RC, Vantrease JE, Love S, Padival M, Rosenkranz JA (2017) An intra-amygdala circuit specifically regulates social fear learning. Nature Neurosci 20(3):459–469
Van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM (1999) Play is indispensable for an adequate development of coping with social challenges in the rat. Dev Psychobiol 34:129–138
Van den Berg CL, Pijlman FTA, Koning HAM, Diergaarde L, Van Ree JM, Spruijt BM (1999) Isolation changes the incentive value of sucrose and social behaviour in juvenile and adult rats. Behav Brain Res 106:133–142
Van den Berg CL, Van Ree JM, Spruijt BM, Kitchen I (1999) Effects of juvenile isolation and morphine treatment on social interactions and opioid receptors in adult rats: behavioural and autoradiographic studies. Eur J Neurosci 11:3023–3032
Van den Berg CL, Van Ree JM, Spruijt BM (2000) Morphine attenuates the effects of juvenile isolation in rats. Neuropharmacol 39:969–976
Vanderschuren LJMJ, Trezza V (2014) What the laboratory rat has taught us about social play behavior: role in behavioral development and neural mechanisms. Curr Top Behav Neurosci 16:189–212
Vanderschuren LJMJ, Niesink RJM, Spruijt BM, Van Ree JM (1995) Effects of morphine on different aspects of social play in juvenile rats. Psychopharmacol 117:225–231
Vanderschuren LJMJ, Trezza V, Griffioen-Roose S, Schiepers OJG, Van Leeuwen N, De Vries TJ, Schoffelmeer ANM (2008) Methylphenidate disrupts social play behavior in adolescent rats. Neuropsychopharmacol 33:2946–2956
Vanderschuren LJMJ, Achterberg EJM, Trezza V (2016) The neurobiology of social play and its rewarding value in rats. Neuroscience and Biobehavioral Reviews 70:86–105
Varlinskaya EI, Spear LP (2008) Social interactions in adolescent and adult Sprague-Dawley rats: Impact of social deprivation and test context familiarity. Behav Brain Res 188(2):398–405
Veeneman MMJ, Boleij H, Broekhoven MH, Snoeren EMS, Guitart Masip M, Cousijn J, Vanderschuren LJMJ (2011) Dissociable roles of mGlu5 and dopamine receptors in the rewarding and sensitizing properties of morphine and cocaine. Psychopharmacol 214:863–876
Von Frijtag JC, Schot M, van den Bos R, Spruijt BM (2002) Individual housing during the play period results in changed responses to and consequences of a psychosocial stress situation in rats. Dev Psychobiol 41:58–69
Wang D, Noda Y, Zhou Y, Nitta A, Furukawa H, Nabeshima T (2007) Synergistic effect of galantamine with risperidone on impairment of social interaction in phencyclidine-treated mice as a schizophrenic animal model. Neuropharm 52(4):1179–1187
Whitaker LR, Degoulet M, Morikawa H (2013) Social deprivation enhances VTA synaptic plasticity and drug-induced contextual learning. Neuron 77:335–345
Wright IK, Upton N, Marsden CA (1991) Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol Behav 50:129–132
Wu D, Zhu J, You L, Wang J, Zhang S, Liu Z, Xu Q, Yuan X, Yang L, Wang W, Tong M, Hong Q, Chi X (2023) NRXN1 depletion in the medial prefrontal cortex induces anxiety-like behaviors and abnormal social phenotypes along with impaired neurite outgrowth in rat. J Neurodev Disord 15:6
Yizhar O, Fenno LE, Prigge M, Hegemann P, Deisseroth K (2011) Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477(7363):171–178
Acknowledgements
We would like to thank Judith Hendriks, Ruth Damsteegt, Joep Titulaer, Nina Driel, Audry Genet and Martine Maco for their assistance with the breeding colony, genotyping animals and behavioural experiments.
Funding
M. Achterberg is supported by the Roche Postdoctoral Fellowship Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to a Special Issue on Spanning the spectrum of social behavior: towards more translationally relevant animal models
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Achterberg, E.J.M., Biemans, B. & Vanderschuren, L.J.M.J. Neurexin1α knockout in rats causes aberrant social behaviour: relevance for autism and schizophrenia. Psychopharmacology (2024). https://doi.org/10.1007/s00213-024-06559-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00213-024-06559-z