Abstract
Rationale
Impaired N-methyl-d-aspartate (NMDA) receptor signalling underlies several psychiatric disorders that express high levels of impulsivity. Although synergistic interactions exist between NMDA receptors and metabotropic glutamate receptor 5 (mGluR5), the significance of this interaction for impulsivity is unknown.
Objective
This study aims to investigate the effects of negative and positive allosteric mGluR5 modulation (NAM/PAM) on trait impulsivity and impulsivity evoked by NMDA receptor antagonism in rats.
Methods
Motor and choice impulsivity were assessed using the five-choice serial reaction time task (5-CSRTT) and delayed-discounting task (DDT), respectively. The effects of RO4917523 and 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) (NAMs) and ADX47273 (PAM) were investigated in non-impulsive rats and in trait high- and low-impulsive rats. The effects of these compounds on impulsivity induced by NMDA receptor antagonism (MK801) in the 5-CSRTT were also investigated.
Results
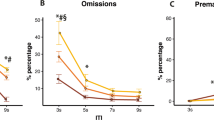
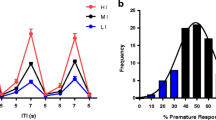
RO4917523 (0.1–1 mg/kg) decreased premature responding and increased omissions but had no effect on locomotor activity up to 0.1 mg/kg. MTEP significantly increased omissions, decreased accuracy and slowed responding but had no effect on premature responding. ADX47273 decreased premature responding at doses that had no effect on locomotor activity. MK801 increased premature responding and impaired attentional accuracy; these deficits were dose dependently rescued by ADX47273 pre-treatment. Allosteric modulation of mGluR5 had no significant effect on choice impulsivity, nor did it modulate general task performance.
Conclusions
These findings demonstrate that mGluR5 allosteric modulation selectively dissociates motor and choice impulsivity. We further show that mGluR5 PAMs may have therapeutic utility in selectively targeting specific aspects of impulsivity and executive dysfunction.







Similar content being viewed by others
References
Adams B, Moghaddam B (1998) Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci 18:5545–5554
Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, Breier A (1999) Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry 156:1646–1649
Agnoli L, Carli M (2012) Dorsal–striatal 5-HT2A and 5-HT2C receptors control impulsivity and perseverative responding in the 5-choice serial reaction time task. Psychopharmacology 219:633–645. doi:10.1007/s00213-011-2581-0
Ahnaou A, Langlois X, Steckler T, Bartolome-Nebreda JM, Drinkenburg WHIM (2015) Negative versus positive allosteric modulation of metabotropic glutamate receptors (mGluR5): indices for potential pro-cognitive drug properties based on EEG network oscillations and sleep-wake organization in rats. Psychopharmacology 232:1107–1122. doi:10.1007/s00213-014-3746-4
Ainslie G (1975) Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull 82:463–496
Anderson JJ, Bradbury MJ, Giracello DR, Chapman DF, Holtz G, Roppe J, King C, Cosford NDP, Varney MA (2003) In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligand [3H]3-methoxy-5-(pyridin-2-ylethynyl)pyridine). Eur J Pharmacol 473:35–40
Aron AR, Poldrack RA (2005) The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry 57:1285–1292. doi:10.1016/j.biopsych.2004.10.026
Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ (2000) Activation of metabotropic glutamate receptor 5 Has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci 20:7871–7879
Bari A, Dalley JW, Robbins TW (2008) The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protocols 3:759–767. doi:10.1038/nprot.2008.41
Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ (2008) High impulsivity predicts the switch to compulsive cocaine-taking. Science 320:1352–1355
Busse CS, Brodkin J, Tattersall D, Anderson JJ, Warren N, Tehrani L, Bristow LJ, Varney MA, Cosford ND (2004) The behavioral profile of the potent and selective mGlu5 receptor antagonist 3-[lsqb](2-methyl-1,3-thiazol-4-yl)ethynyl[rsqb]pyridine (MTEP) in rodent models of anxiety. Neuropsychopharmacology 29:1971–1979
Campbell UC, Lalwani K, Hernandez L, Kinney GG, Conn PJ, Bristow LJ (2004) The mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) potentiates PCP-induced cognitive deficits in rats. Psychopharmacology (Berl) 175:310–318. doi:10.1007/s00213-004-1827-5
Cardinal RN, Aitken MRF (2010) Whisker: a client-server high-performance multimedia research control system. Behav Res Methods 42:1059–1071. doi:10.3758/BRM.42.4.1059
Cardinal RN, Pennicott DR, Lakmali C, Robbins TW, Sugathapala CL, Everitt BJ (2001) Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science 292:2499–2501
Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ (2004) Limbic corticostriatal systems and delayed reinforcement. Ann N Y Acad Sci 1021:33–50. doi:10.1196/annals.1308.004
Carli M, Robbins TW, Evenden JL, Everitt BJ (1983) Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res 9:361–380
Carli M, Baviera M, Invernizzi R, Balducci C (2004) The Serotonin 5-HT2A Receptors Antagonist M100907 prevents impairment in attentional performance by NMDA receptor blockade in the rat prefrontal cortex. Neuropsychopharmacology 29. doi:10.1038/sj.npp.1300479
Ceglia I, Carli M, Baviera M, Renoldi G, Calcagno E, Invernizzi RW (2004) The 5-HT2A receptor antagonist M100,907 prevents extracellular glutamate rising in response to NMDA receptor blockade in the mPFC. J Neurochem 91:189–199. doi:10.1111/j.1471-4159.2004.02704.x
Chu Z, Hablitz JJ (1998) Activation of group I mGluRs increases spontaneous IPSC frequency in rat frontal cortex. J Neurophysiol 80:621–627
Chudasama Y, Baunez C, Robbins TW (2003) Functional disconnection of the medial prefrontal cortex and subthalamic nucleus in attentional performance: evidence for corticosubthalamic interaction. J Neurosci 23:5477–5485
Clifton NE, Morisot N, Girardon S, Millan MJ, Loiseau F (2013) Enhancement of social novelty discrimination by positive allosteric modulators at metabotropic glutamate 5 receptors: adolescent administration prevents adult-onset deficits induced by neonatal treatment with phencyclidine. Psychopharmacology (Berl) 225:579–594. doi:10.1007/s00213-012-2845-3
Cole BJ, Robbins TW (1987) Amphetamine impairs the discriminative performance of rats with dorsal noradrenergic bundle lesions on a 5-choice serial reaction time task: new evidence for central dopaminergic-noradrenergic interactions. Psychopharmacology 91:458–466. doi:10.1007/BF00216011
Conn PJ, Pin JP (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37:205–237. doi:10.1146/annurev.pharmtox.37.1.205
Cosford NDP, Roppe J, Tehrani L, Schweiger EJ, Seiders TJ, Chaudary A, Rao S, Varney MA (2003) [3H]-Methoxymethyl-MTEP and [3H]-Methoxy-PEPy: potent and selective radioligands for the metabotropic glutamate subtype 5 (mGlu5) receptor. Bioorg Med Chem Lett 13:351–354
Crunelle CL, Veltman DJ, van Emmerik-van Oortmerssen K, Booij J, van den Brink W (2013) Impulsivity in adult ADHD patients with and without cocaine dependence. Drug Alcohol Depend 129:18–24. doi:10.1016/j.drugalcdep.2012.09.006
Dalley JW, Roiser JP (2012) Dopamine, serotonin and impulsivity. Neuroscience 215:42–58. doi:10.1016/j.neuroscience.2012.03.065
Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron J-C, Everitt BJ, Robbins TW (2007) Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315:1267–1270. doi:10.1126/science.1137073
Dalley JW, Everitt BJ, Robbins TW (2011) Impulsivity, compulsivity, and top–down cognitive control. Neuron 69:680–694. doi:10.1016/j.neuron.2011.01.020
Darrah JM, Stefani MR, Moghaddam B (2008) Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behav Pharmacol 19:225–234. doi:10.1097/FBP.0b013e3282feb0ac
De Paulis T, Hemstapat K, Chen Y, Zhang Y, Saleh S, Alagille D, Baldwin RM, Tamagnan GD, Conn PJ (2006) Substituent effects of N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamides on positive allosteric modulation of the metabotropic glutamate-5 receptor in Rat cortical astrocytes. J Med Chem 49:3332–3344. doi:10.1021/jm051252j
De Wit H (2009) Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol 14:22–31. doi:10.1111/j.1369-1600.2008.00129.x
Deakin JFW, Slater P, Simpson MDC, Gilchrist AC, Skan WJ, Royston MC, Reynolds GP, Cross AJ (1989) Frontal cortical and left temporal glutamatergic dysfunction in schizophrenia. J Neurochem 52:1781–1786. doi:10.1111/j.1471-4159.1989.tb07257.x
Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer ANM, De Vries TJ (2008) Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry 63:301–308. doi:10.1016/j.biopsych.2007.07.011
Doherty A, Palmer M, Henley J, Collingridge G, Jane D (1997) (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) activates mGlu5, but not mGlu1, receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus. Neuropharmacology 36:265–267. doi:10.1016/S0028-3908(97)00001-4
Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ (2009) High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry 65:851–856. doi:10.1016/j.biopsych.2008.12.008
Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW (2010) Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol Psychiatry 68:770–773. doi:10.1016/j.biopsych.2010.06.015
Evenden JL (1999) Varieties of impulsivity. Psychopharmacology 146:348–361. doi:10.1007/PL00005481
Fletcher PJ, Rizos Z, Noble K, Higgins GA (2011) Impulsive action induced by amphetamine, cocaine and MK801 is reduced by 5-HT2C receptor stimulation and 5-HT2A receptor blockade. Neuropharmacology 61:468–477. doi:10.1016/j.neuropharm.2011.02.025
Fowler SW, Ramsey AK, Walker JM, Serfozo P, Olive MF, Schachtman TR, Simonyi A (2011) Functional interaction of mGlu5 and NMDA receptors in aversive learning in rats. Neurobiol Learn Mem 95:73–79. doi:10.1016/j.nlm.2010.11.009
Gaalen M, Brueggeman R, Bronius PC, Schoffelmeer AM, Vanderschuren LMJ (2006) Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology 187:73–85. doi:10.1007/s00213-006-0396-1
Gass JT, Osborne MPH, Watson NL, Brown JL, Olive MF (2008) mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology 34:820–833
Gastambide F, Gilmour G, Robbins TW, Tricklebank MD (2013) The mGlu5 positive allosteric modulator LSN2463359 differentially modulates motor, instrumental and cognitive effects of NMDA receptor antagonists in the rat. Neuropharmacology 64:240–247. doi:10.1016/j.neuropharm.2012.07.039
Goff DC, Coyle JT (2001) The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry 158:1367–1377
Greco B, Invernizzi R, Carli M (2005) Phencyclidine-induced impairment in attention and response control depends on the background genotype of mice: reversal by the mGLU2/3 receptor agonist LY379268. Psychopharmacology 179:68–76. doi:10.1007/s00213-004-2127-9
Gupta DS, McCullumsmith RE, Beneyto M, Haroutunian V, Davis KL, Meador-Woodruff JH (2005) Metabotropic glutamate receptor protein expression in the prefrontal cortex and striatum in schizophrenia. Synapse 57:123–131. doi:10.1002/syn.20164
Harvey BD, Siok CJ, Kiss T, Volfson D, Grimwood S, Shaffer CL, Hajós M (2013) Neurophysiological signals as potential translatable biomarkers for modulation of metabotropic glutamate 5 receptors. Neuropharmacology 75:19–30. doi:10.1016/j.neuropharm.2013.06.020
Heidbreder CA, Bianchi M, Lacroix LP, Faedo S, Perdona E, Remelli R, Cavanni P, Crespi F (2003) Evidence that the metabotropic glutamate receptor 5 antagonist MPEP may act as an inhibitor of the norepinephrine transporter in vitro and in vivo. Synapse 50:269–276. doi:10.1002/syn.10261
Henry S, Lehmann-Masten V, Gasparini F, Geyer M, Markou A (2002) The mGluR5 antagonist MPEP, but not the mGluR2/3 agonist LY314582, augments PCP effects on prepulse inhibition and locomotor activity. Neuropharmacology 43:1199–1209. doi:10.1016/S0028-3908(02)00332-5
Hester R, Garavan H (2004) Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci 24:11017–11022. doi:10.1523/JNEUROSCI.3321-04.2004
Higgins GA, Enderlin M, Haman M, Haman PI (2003) The 5-HT2A receptor antagonist M100,907 attenuates motor and “impulsive-type” behaviours produced by NMDA receptor antagonism. Psychopharmacology 170:309–319. doi:10.1007/s00213-003-1549-0
Homayoun H, Moghaddam B (2006) Bursting of prefrontal cortex neurons in awake rats is regulated by metabotropic glutamate 5 (mGlu5) receptors: rate-dependent influence and interaction with NMDA receptors. Cereb Cortex 16:93–105. doi:10.1093/cercor/bhi087
Homayoun H, Moghaddam B (2007) NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27:11496–11500
Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B (2004) Functional interaction between NMDA and mGlu5 receptors: effects on working memory, instrumental learning, motor behaviors, and dopamine release. Neuropsychopharmacology 29:1259–1269
Jackson ME, Homayoun H, Moghaddam B (2004) NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci U S A 101:8467–8472
Jaeschke G, Kolczewski S, Spooren W, Vieira E, Bitter-Stoll N, Boissin P, Borroni E, Büttelmann B, Ceccarelli S, Clemann N, David B, Funk C, Guba W, Harrison A, Hartung T, Honer M, Huwyler J, Kuratli M, Niederhauser U, Pähler A, Peters J-U, Petersen A, Prinssen E, Ricci A, Rueher D, Rueher M, Schneider M, Spurr P, Stoll T, Tännler D, Wichmann J, Porter RH, Wettstein JG, Lindemann L (2015) Metabotropic glutamate receptor 5 negative allosteric modulators: discovery of 2-chloro-4-[1-(4-fluorophenyl)-2,5-dimethyl-1H-imidazol-4-ylethynyl]-pyridine (Basimglurant, RO4917523), a promising novel medicine for psychiatric diseases. J Med Chem. doi:10.1021/jm501642c
Kaladjian A, Jeanningros R, Azorin J-M, Anton J-L, Mazzola-Pomietto P (2011) Impulsivity and neural correlates of response inhibition in schizophrenia. Psychol Med 41:291–299. doi:10.1017/S0033291710000796
Kinney GG, Burno M, Campbell UC, Hernandez LM, Rodriguez D, Bristow LJ, Conn PJ (2003) Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents. J Pharmacol Exp Ther 306:116–123. doi:10.1124/jpet.103.048702
Konradi C, Heckers S (2003) Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol Ther 97:153–179. doi:10.1016/S0163-7258(02)00328-5
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214
Lahti AC, Weiler MA, Michaelidis T, Parwani A, Tamminga CA (2001) Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology 25:455–467
Lea PM, Faden AI (2006) Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev 12:149–166. doi:10.1111/j.1527-3458.2006.00149.x
Lecourtier L, Homayoun H, Tamagnan G, Moghaddam B (2007) Positive allosteric modulation of metabotropic glutamate 5 (mGlu5) receptors reverses N-methyl-D-aspartate antagonist-induced alteration of neuronal firing in prefrontal cortex. Biol Psychiatry 62:739–746. doi:10.1016/j.biopsych.2006.12.003
Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA (2009) Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci 29:14734–14740. doi:10.1523/JNEUROSCI.3765-09.2009
Lindsley CW, Shipe WD, Wolkenberg SE, Theberge CR, Williams DL Jr, Sur C, Kinney GG (2006) Progress towards validating the NMDA receptor hypofunction hypothesis of schizophrenia. Curr Top Med Chem 6:771–785
Liu F, Grauer S, Kelley C, Navarra R, Graf R, Zhang G, Atkinson PJ, Popiolek M, Wantuch C, Khawaja X, Smith D, Olsen M, Kouranova E, Lai M, Pruthi F, Pulicicchio C, Day M, Gilbert A, Pausch MH, Brandon NJ, Beyer CE, Comery TA, Logue S, Rosenzweig-Lipson S, Marquis KL (2008) ADX47273 [S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piperidin-1-yl}-methanone]: a novel metabotropic glutamate receptor 5-selective positive allosteric modulator with preclinical antipsychotic-like and procognitive activities. J Pharmacol Exp Ther 327:827–839
Luby C (1959) STudy of a new schizophrenomimetic drug—sernyl. A.M.A. Arch Neurol Psychiatry 81:363–369. doi:10.1001/archneurpsyc.1959.02340150095011
Mar AC, Robbins TW (2007) Delay discounting and impulsive choice in the rat. Curr Protoc Neurosci Chapter 8, Unit 8.22. doi:10.1002/0471142301.ns0822s39
Mar AC, Walker ALJ, Theobald DE, Eagle DM, Robbins TW (2011) Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J Neurosci 31:6398–6404. doi:10.1523/JNEUROSCI.6620-10.2011
Mobini S, Body S, Ho M-Y, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM (2002) Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 160:290–298. doi:10.1007/s00213-001-0983-0
Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC (2001a) Psychiatric aspects of impulsivity. Am J Psychiatry 158:1783–1793. doi:10.1176/appi.ajp.158.11.1783
Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J (2001b) The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat 21:193–198
Moghaddam B, Adams BW (1998) Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281:1349–1352
Moghaddam B, Adams B, Verma A, Daly D (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17:2921–2927
Muir JL, Everitt BJ, Robbins TW (1996) The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex 6:470–481. doi:10.1093/cercor/6.3.470
Murphy E, Dalley J, Robbins T (2005) Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology 179:99–107. doi:10.1007/s00213-004-2068-3
Murphy E, Fernando AP, Urcelay G, Robinson EJ, Mar A, Theobald DH, Dalley J, Robbins T (2012) Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology 219:401–410. doi:10.1007/s00213-011-2572-1
Nakanishi S (1992) Molecular diversity of glutamate receptors and implications for brain function. Science 258:597–603. doi:10.1126/science.1329206
Nakanishi S, Masu M (1994) Molecular diversity and functions of glutamate receptors. Annu Rev Biophys Biomol Struct 23:319–348. doi:10.1146/annurev.bb.23.060194.001535
Nordquist RE, Durkin S, Jaeschke G, Spooren W (2007) Stress-induced hyperthermia: effects of acute and repeated dosing of MPEP. Eur J Pharmacol 568:199–202. doi:10.1016/j.ejphar.2007.04.034
Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA Jr (2007) Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in Sprague–Dawley rats. Biol Psychiatry 62:687–693. doi:10.1016/j.biopsych.2006.11.017
Pattij T, Vanderschuren LJMJ (2008) The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci 29:192–199. doi:10.1016/j.tips.2008.01.002
Pezze MA, Dalley JW, Robbins TW (2009) Remediation of attentional dysfunction in rats with lesions of the medial prefrontal cortex by intra-accumbens administration of the dopamine D2/3 receptor antagonist sulpiride. Psychopharmacology 202:307–313. doi:10.1007/s00213-008-1384-4
Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P (2001) Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience 106:579–587. doi:10.1016/S0306-4522(01)00297-4
Porter RHP, Jaeschke G, Spooren W, Ballard TM, Büttelmann B, Kolczewski S, Peters J-U, Prinssen E, Wichmann J, Vieira E, Mühlemann A, Gatti S, Mutel V, Malherbe P (2005) Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J Pharmacol Exp Ther 315:711–721. doi:10.1124/jpet.105.089839
Pozzi L, Baviera M, Sacchetti G, Calcagno E, Balducci C, Invernizzi RW, Carli M (2011) Attention deficit induced by blockade of N-methyl D-aspartate receptors in the prefrontal cortex is associated with enhanced glutamate release and cAMP response element binding protein phosphorylation: role of metabotropic glutamate receptors 2/3. Neuroscience 176:336–348. doi:10.1016/j.neuroscience.2010.11.060
Robbins T (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology 163:362–380. doi:10.1007/s00213-002-1154-7
Romano C, Sesma MA, McDonald CT, O’malley K, van den Pol AN, Olney JW (1995) Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol 355:455–469. doi:10.1002/cne.903550310
Rosenbrock H, Kramer G, Hobson S, Koros E, Grundl M, Grauert M, Reymann KG, Schröder UH (2010) Functional interaction of metabotropic glutamate receptor 5 and NMDA-receptor by a metabotropic glutamate receptor 5 positive allosteric modulator. Eur J Pharmacol 639:40–46. doi:10.1016/j.ejphar.2010.02.057
Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MFS (2006) Separate neural pathways process different decision costs. Nat Neurosci 9:1161–1168. doi:10.1038/nn1756
Schlumberger C, Pietraszek M, Gravius A, Klein K-U, Greco S, Morè L, Danysz W (2009) Comparison of the mGlu5 receptor positive allosteric modulator ADX47273 and the mGlu2/3 receptor agonist LY354740 in tests for antipsychotic-like activity. Eur J Pharmacol 623:73–83. doi:10.1016/j.ejphar.2009.09.006
Schoepp DD (2001) Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther 299:12–20
Schoepp DD, Conn PJ (2002) Metabotropic glutamate receptors. Pharmacol, Biochem Behav 74:255–256. doi:10.1016/S0091-3057(02)00953-X
Semenova S, Markou A (2007) The effects of the mGluR5 antagonist MPEP and the mGluR2/3 antagonist LY341495 on rats’ performance in the 5-choice serial reaction time task. Neuropharmacology 52:863–872. doi:10.1016/j.neuropharm.2006.10.003
Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N (1993) Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett 163:53–57. doi:10.1016/0304-3940(93)90227-C
Stefani MR, Moghaddam B (2010) Activation of type 5 metabotropic glutamate receptors attenuates deficits in cognitive flexibility induced by NMDA receptor blockade. Eur J Pharmacol 639:26–32. doi:10.1016/j.ejphar.2010.01.028
Sukhotina IA, Dravolina OA, Novitskaya Y, Zvartau EE, Danysz W, Bespalov AY (2008) Effects of mGlu1 receptor blockade on working memory, time estimation, and impulsivity in rats. Psychopharmacology (Berl) 196:211–220. doi:10.1007/s00213-007-0953-2
Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JSH, McNaughton CH, Jacobson MA, Hutson PH (2009) Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology 57:531–538. doi:10.1016/j.neuropharm.2009.07.022
Varty G, Grilli M, Forlani A, Fredduzzi S, Grzelak M, Guthrie D, Hodgson R, Lu S, Nicolussi E, Pond A, Parker E, Hunter J, Higgins G, Reggiani A, Bertorelli R (2005) The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists, MPEP and MTEP, and the mGluR1 antagonist, LY456236, in rodents: a comparison of efficacy and side-effect profiles. Psychopharmacology 179:207–217. doi:10.1007/s00213-005-2143-4
Winstanley CA, Dalley JW, Theobald DEH, Robbins TW (2003) Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology (Berl) 170:320–331. doi:10.1007/s00213-003-1546-3
Winstanley CA, Eagle DM, Robbins TW (2006) Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev 26:379–395. doi:10.1016/j.cpr.2006.01.001
Yates JR, Batten SR, Bardo MT, Beckmann JS (2014) Role of ionotropic glutamate receptors in delay and probability discounting in the rat. Psychopharmacology (Berl). doi:10.1007/s00213-014-3747-3
Yonezawa Y, Kuroki T, Kawahara T, Tashiro N, Uchimura H (1998) Involvement of γ-aminobutyric acid neurotransmission in phencyclidine-induced dopamine release in the medial prefrontal cortex. Eur J Pharmacol 341:45–56. doi:10.1016/S0014-2999(97)01435-0
Acknowledgements
This research was supported by a Medical Research Council (MRC) grant to JWD (G0701500) and a grant from Boehringer Ingelheim Pharma GmbH & Co. KG. This work was carried out in the Behavioural and Clinical Neuroscience Institute (BCNI) at Cambridge University with joint support from the MRC (G1000183) and Wellcome Trust (093875/Z/10/Z) and at Boehringer Ingelheim Pharma GmbH & Co. KG, Germany. We thank David Theobald, Johannes Freudenreich, Peter Schorn, Alfie Wearn and Benjamin Jaehnke for technical support and Gert Kramer, Dr. Holger Rosenbrock and Dr. Cornelia Dorner-Ciossek for helpful scientific discussions. The authors declare that the experiments performed in this manuscript followed the principles of laboratory animal care and are in compliance with the current laws of the UK and Germany.
Conflict of interest
The authors declare no conflict of interest.
Sarah Isherwood, Anton Pekcec and Janet Nicholson are employees of Boehringer Ingelheim Pharma GmbH & Co. KG
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 777 kb)
Rights and permissions
About this article
Cite this article
Isherwood, S.N., Pekcec, A., Nicholson, J.R. et al. Dissociable effects of mGluR5 allosteric modulation on distinct forms of impulsivity in rats: interaction with NMDA receptor antagonism. Psychopharmacology 232, 3327–3344 (2015). https://doi.org/10.1007/s00213-015-3984-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-3984-0