Abstract
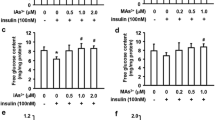
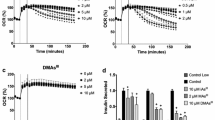
Inorganic arsenic (iAs) is an environmental diabetogen, but mechanisms underlying its diabetogenic effects are poorly understood. Exposures to arsenite (iAsIII) and its methylated metabolites, methylarsonite (MAsIII) and dimethylarsinite (DMAsIII), have been shown to inhibit glucose-stimulated insulin secretion (GSIS) in pancreatic β-cells and isolated pancreatic islets. GSIS is regulated by complex mechanisms. Increase in ATP production through metabolism of glucose and other substrates is the ultimate trigger for GSIS in β-cells. In the present study, we used metabolomics to identify metabolites and pathways perturbed in cultured INS-1 832/13 rat insulinoma cells and isolated murine pancreatic islets by exposures to iAsIII, MAsIII and DMAsIII. We found that the exposures perturbed multiple metabolites, which were enriched primarily in the pathways of amino acid, carbohydrate, phospholipid and carnitine metabolism. However, the effects of arsenicals in INS-1 832/13 cells differed from those in the islets and were exposure specific with very few overlaps between the three arsenicals. In INS-1 832/13 cells, all three arsenicals decreased succinate, a metabolite of Krebs cycle, which provides substrates for ATP synthesis in mitochondria. Acetylcarnitine was decreased consistently by exposures to arsenicals in both the cells and the islets. Acetylcarnitine is usually found in equilibrium with acetyl-CoA, which is the central metabolite in the catabolism of macronutrients and the key substrate for Krebs cycle. It is also thought to play an antioxidant function in mitochondria. Thus, while each of the three trivalent arsenicals perturbed specific metabolic pathways, which may or may not be associated with GSIS, all three arsenicals appeared to impair mechanisms that support ATP production or antioxidant defense in mitochondria. These results suggest that impaired ATP production and/or mitochondrial dysfunction caused by oxidative stress may be the mechanisms underlying the inhibition of GSIS in β-cells exposed to trivalent arsenicals.







Similar content being viewed by others
References
Aichler M, Borgmann D, Krumsiek J, Buck A, MacDonald PE, Fox JEM, Lyon J, Light PE, Keipert S, Jastroch M, Feuchtinger A, Mueller NS, Sun N, Palmer A, Alexandrov T, Hrabe de Angelis M, Neschen S, Tschop MH, Walch A (2017) N-acyl taurines and acylcarnitines cause an imbalance in insulin synthesis and secretion provoking beta cell dysfunction in type 2 diabetes. Cell Metab 25(6):1334–1347
ATSDR (2007) Toxicological profile for arsenic. DHHS, Public Health Service, Agency for Toxic Substances and Disease Registry, US
Auerbach S, Filer D, Reif D, Walker V, Holloway AC, Schlezinger J, Srinivasan S, Svoboda D, Judson R, Bucher JR, Thayer KA (2016) Prioritizing environmental chemicals for obesity and diabetes outcomes research: a screening approach using ToxCast high-throughput data. Environ Health Perspect 124(8):1141–1154
Beck R, Styblo M, Sethupathy P (2017) Arsenic exposure and type 2 diabetes: MicroRNAs as mechanistic links? Curr Diab Rep 17(3):18
Beck R, Chandi M, Kanke M, Styblo M, Sethupathy P (2019) Arsenic is more potent than cadmium or manganese in disrupting the INS-1 beta cell microRNA landscape. Arch Toxicol 93(11):3099–3109
Bodaghi-Namileh V, Sepand MR, Omidi A, Aghsami M, Seyednejad SA, Kasirzadeh S, Sabzevari O (2018) Acetyl-l-carnitine attenuates arsenic-induced liver injury by abrogation of mitochondrial dysfunction, inflammation, and apoptosis in rats. Environ Toxicol Pharmacol 58:11–20
Bonini MG, Sargis RM (2018) Environmental toxicant exposures and type 2 diabetes mellitus: two interrelated public health problems on the rise. Curr Opin Toxicol 7:52–59
Calabrese V, Giuffrida Stella AM, Calvani M, Butterfield DA (2006) Acetylcarnitine and cellular stress response: roles in nutritional redox homeostasis and regulation of longevity genes. J Nutr Biochem 17(2):73–88
Chen G, Hohmeier HE, Gasa R, Tran VV, Newgard CB (2000) Selection of insulinoma cell lines with resistance to interleukin-1beta- and gamma-interferon-induced cytotoxicity. Diabetes 49(4):562–570
Cresto JC, Fabiano de Bruno LE, Cao GF, Pastorale CF, Confalonieri N, del Carmen CM, Basabe JC (2006) The association of acetyl-l-carnitine and nicotinamide remits the experimental diabetes in mice by multiple low-dose streptozotocin. Pancreas 33(4):403–411
Cubadda F, Jackson BP, Cottingham KL, Van Horne YO, Kurzius-Spencer M (2017) Human exposure to dietary inorganic arsenic and other arsenic species: state of knowledge, gaps and uncertainties. Sci Total Environ 579:1228–1239
Diaz-Villasenor A, Sanchez-Soto MC, Cebrian ME, Ostrosky-Wegman P, Hiriart M (2006) Sodium arsenite impairs insulin secretion and transcription in pancreatic βs. Toxicol Appl Pharmacol 214(1):30–34
CDC (2017) National Diabetes Statistics Report, 2017. Estimates of Diabetes and Its Burden in the United States. National Center for Chronic Disease Prevention and Health Promotion, Division of Diabetes Translation
Douillet C, Currier J, Saunders J, Bodnar WM, Matousek T, Styblo M (2013) Methylated trivalent arsenicals are potent inhibitors of glucose stimulated insulin secretion by murine pancreatic islets. Toxicol Appl Pharmacol 267(1):11–15
Dover EN, Beck R, Huang MC, Douillet C, Wang Z, Klett EL, Styblo M (2018a) Arsenite and methylarsonite inhibit mitochondrial metabolism and glucose-stimulated insulin secretion in INS-1 832/13 beta cells. Arch Toxicol 92(2):693–704
Dover EN, Patel NY, Styblo M (2018b) Impact of in vitro heavy metal exposure on pancreatic beta-cell function. Toxicol Lett 299:137–144
Dunn WB, Broadhurst D, Begley P, Zelena E, Francis-McIntyre S, Anderson N, Brown M, Knowles JD, Halsall A, Haselden JN, Nicholls AW (2011) Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protoc 6(7):1060–1083
Esko JD, Kimata K, Lindahl U (2009) Proteoglycans and sulfated glycosaminoglycans. In: Varki A, Cummings RD, et al. (eds) Essentials of glycobiology. Cold Spring Harbor, New York
Farrell S, Vogel J, Bieber LL (1986) Entry of acetyl-l-carnitine into biosynthetic pathways. Biochim Biophys Acta 876(1):175–177
Folli F, La Rosa S, Finzi G, Davalli AM, Galli A, Dick EJ Jr, Perego C, Mendoza RG (2018) Pancreatic islet of Langerhans' cytoarchitecture and ultrastructure in normal glucose tolerance and in type 2 diabetes mellitus. Diabetes Obes Metab 20(Suppl 2):137–144
Fu J, Woods CG, Yehuda-Shnaidman E, Zhang Q, Wong V, Collins S, Sun G, Andersen ME, Pi J (2010) Low-level arsenic impairs glucose-stimulated insulin secretion in pancreatic beta cells: involvement of cellular adaptive response to oxidative stress. Environ Health Perspect 118(6):864–870
Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB (2000) Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49(3):424–430
Huang M, Douillet C, Styblo M (2019) Arsenite and its trivalent methylated metabolites inhibit glucose-stimulated calcium influx and insulin secretion in murine pancreatic islets. Arch Toxicol 93(9):2525–2533
IARC (2004) Some Drinking-water disinfectants and contaminants, including arsenic, vol 84. WHO, International Agency for Research of Cancer, Geneva, Switzerland
IDF (2015) IDF diabetes atlas, 7th Edition, www.diabetesatlas.org.edn. International Diabetes Federation
Iossa S, Mollica MP, Lionetti L, Crescenzo R, Botta M, Barletta A, Liverini G (2002) Acetyl-l-carnitine supplementation differently influences nutrient partitioning, serum leptin concentration and skeletal muscle mitochondrial respiration in young and old rats. J Nutr 132(4):636–642
Keshavarz-Bahaghighat H, Sepand MR, Ghahremani MH, Aghsami M, Sanadgol N, Omidi A, Bodaghi-Namileh V, Sabzevari O (2018) Acetyl-l-carnitine attenuates arsenic-induced oxidative stress and hippocampal mitochondrial dysfunction. Biol Trace Elem Res 184(2):422–435
Khan F, Momtaz S, Niaz K, Hassan FI, Abdollahi M (2017) Epigenetic mechanisms underlying the toxic effects associated with arsenic exposure and the development of diabetes. Food Chem Toxicol 107(Pt A):406–417
Kuo CC, Moon KA, Wang SL, Silbergeld E, Navas-Acien A (2017) The association of arsenic metabolism with cancer, cardiovascular disease, and diabetes: a systematic review of the epidemiological evidence. Environ Health Perspect 125(8):087001
Lorenz MA, Burant CF, Kennedy RT (2011) Reducing time and increasing sensitivity in sample preparation for adherent mammalian cell metabolomics. Anal Chem 83(9):3406–3414
Mano ECC, Scott AL, Honorio KM (2018) UDP-glucuronosyltransferases: structure, function and drug design studies. Curr Med Chem 25(27):3247–3255
Martin EM, Styblo M, Fry RC (2017) Genetic and epigenetic mechanisms underlying arsenic-associated diabetes mellitus: a perspective of the current evidence. Epigenomics 9(5):701–710
Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, Silbergeld EK, Styblo M, Tseng CH, Thayer KA, Loomis D (2012) Evaluation of the association between arsenic and diabetes: a national toxicology program workshop review. Environ Health Perspect 120(12):1658–1670
Meng DH, Du RR, Chen LZ, Li MT, Liu F, Hou J, Shi YK, Wang FS, Sheng JZ (2019) Cascade synthesis of uridine-5'-diphosphate glucuronic acid by coupling multiple whole cells expressing hyperthermophilic enzymes. Microb Cell Fact 18(1):118
Rorsman P, Eliasson L, Renstrom E, Gromada J, Barg S, Gopel S (2000) The cell physiology of biphasic insulin secretion. News Physiol Sci 15:72–77
Rosca MG, Lemieux H, Hoppel CL (2009) Mitochondria in the elderly: Is acetylcarnitine a rejuvenator? Adv Drug Deliv Rev 61(14):1332–1342
Sepand MR, Razavi-Azarkhiavi K, Omidi A, Zirak MR, Sabzevari S, Kazemi AR, Sabzevari O (2016) Effect of acetyl-l-carnitine on antioxidant status, lipid peroxidation, and oxidative damage of arsenic in rat. Biol Trace Elem Res 171(1):107–115
Spégel P, Sharoyko VV, Goehring I, Danielsson AP, Malmgren S, Nagorny CL, Andersson LE, Koeck T, Sharp GW, Straub SG, Wollheim CB, Mulder H (2013) Time-resolved metabolomics analysis of beta-cells implicates the pentose phosphate pathway in the control of insulin release. Biochem J 450(3):595–605
Sun Q, Yang Q, Xu H, Xue J, Chen C, Yang X, Gao X, Liu Q (2019) miR-149 negative regulation of mafA is involved in the arsenite-induced dysfunction of insulin synthesis and secretion in pancreatic beta cells. Toxicol Sci 167(1):116–125
Sung TC, Huang JW, Guo HR (2015) Association between arsenic exposure and diabetes: a meta-analysis. Biomed Res Int 2015:368087
Thayer KA, Heindel JJ, Bucher JR, Gallo MA (2012) Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect 120(6):779–789
Wang W, Xie Z, Lin Y, Zhang D (2014) Association of inorganic arsenic exposure with type 2 diabetes mellitus: a meta-analysis. J Epidemiol Commun Health 68(2):176–184
WHO (2012) State of the science of endocrine disrupting chemicals—2012. WHO, Geneva
Wu W, Yao X, Jiang L, Zhang Q, Bai J, Qiu T, Yang L, Gao N, Yang G, Liu X, Chen M, Sun X (2018) Pancreatic islet-autonomous effect of arsenic on insulin secretion through endoplasmic reticulum stress-autophagy pathway. Food Chem Toxicol 111:19–26
Yang N, Sun R, Liao X, Aa J, Wang G (2017) UDP-glucuronosyltransferases (UGTs) and their related metabolic cross-talk with internal homeostasis: a systematic review of UGT isoforms for precision medicine. Pharmacol Res 121:169–183
Yao XF, Zheng BL, Bai J, Jiang LP, Zheng Y, Qi BX, Geng CY, Zhong LF, Yang G, Chen M, Liu XF, Sun XC (2015) Low-level sodium arsenite induces apoptosis through inhibiting TrxR activity in pancreatic beta-cells. Environ Toxicol Pharmacol 40(2):486–491
Zelena E, Dunn WB, Broadhurst D, Francis-McIntyre S, Carroll KM, Begley P, Ohagan S, Knowles JD, Halsall A, Wilson ID (2009) Development of a robust and repeatable UPLC-MS method for the long-term metabolomic study of human serum. Anal Chem 81(4):1357–1364
Acknowledgements
This study was supported by the NIEHS grant R01ES022697 (PI, Styblo), the UNC Nutrition Obesity Research Center grant DK056350 from NIDDK (PI, Zeisel), and the NIH Common Fund Grant 1U24DK097193 (PI, Sumner). All raw and normalized analytical data and associated metadata have been uploaded in the publicly accessible NIH Common Fund Metabolomics Data Repository (doi: 0.21228/M8PH4S; Project ID: PR000851). We thank Dr. Wimal Pathmasiri for assistance with the pathway analysis.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, YY., Douillet, C., Huang, M. et al. Exposure to inorganic arsenic and its methylated metabolites alters metabolomics profiles in INS-1 832/13 insulinoma cells and isolated pancreatic islets. Arch Toxicol 94, 1955–1972 (2020). https://doi.org/10.1007/s00204-020-02729-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-020-02729-y