Abstract
Summary
Romosozumab is a novel bone-building drug that reduces fracture risk. This health economic analysis indicates that sequential romosozumab-to-alendronate can be a cost-effective treatment option for postmenopausal women with severe osteoporosis at high risk of fracture.
Purpose
To estimate the cost-effectiveness of sequential treatment with romosozumab followed by alendronate (“romosozumab-to-alendronate”) compared with alendronate alone in patients with severe osteoporosis at high risk of fracture in Sweden.
Methods
A microsimulation model with a Markov structure was used to simulate fractures, costs, and quality-adjusted life years (QALYs), for women treated with romosozumab-to-alendronate or alendronate alone. Patients aged 74 years with a recent major osteoporotic fracture (MOF) were followed from the start of treatment until the age of 100 years or death. Treatment with romosozumab for 12 months was followed by alendronate for up to 48 months or alendronate alone with a maximum treatment duration of 60 months. The analysis had a societal perspective. Efficacy of romosozumab and alendronate were derived from phase III randomized controlled trials. Resource use and unit costs were collected from the literature. Cost-effectiveness was estimated using incremental cost-effectiveness ratio (ICER) with QALYs as effectiveness measures.
Results
The base case analysis showed that sequential romosozumab-to-alendronate treatment was associated with 0.089 additional QALYs at an additional cost of €3002 compared to alendronate alone, resulting in an ICER of €33,732. At a Swedish reference willingness-to-pay per QALY of €60,000, romosozumab-to-alendronate had a 97.9% probability of being cost-effective against alendronate alone. The results were most sensitive to time horizon, persistence assumptions, patient age, and treatment efficacy.
Conclusion
The results of this study indicate that sequential romosozumab-to-alendronate can be a cost-effective treatment option for postmenopausal women with severe osteoporosis at high risk of fracture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis causes 9 million new fractures worldwide each year with rising numbers in many countries [1]. The disease is associated with substantial humanistic and economic burden [1]. In Sweden, osteoporosis is estimated to affect 400,000 women aged 50 years and older in a given year, corresponding to a prevalence rate of 22% [2]. In addition, Sweden has one of the highest incidence fracture rates worldwide [3] and it is estimated that one in two Swedish women over the age of 50 years will suffer a fragility fracture during their remaining lifetime [4].
The association between a prior fracture and the risk for a subsequent fracture is well documented [5, 6]. The risk of subsequent fractures following a first fracture is highest within the first 2 years following the initial fragility fracture and ~ 50% of all fractures occurring after an initial fracture will happen in those first 2 years [7,8,9,10]. This increased risk in the first 2 years following a fracture has been termed “imminent risk” and these patients can be characterized as being at very high fracture risk [6]. Recurrent fractures are burdensome to the healthcare system, since they incur high costs for in- and outpatient care and impact the quality of life and mortality of patients [11]. Despite this, patients who suffer a fragility fracture remain largely untreated [12].
Bisphosphonates are the most common pharmacological treatments for osteoporosis in Sweden with a primary effect to inhibit bone resorption [13]. Romosozumab is a bone-forming sclerostin monoclonal antibody for the treatment of severe osteoporosis in postmenopausal women at high risk for fracture and was approved for use in the Europe in 2019 [14]. To extend the benefit achieved with romosozumab treatment, the recommendation is to use romosozumab in a treatment sequence, where 210-mg romosozumab is administered subcutaneously once monthly for 12 months followed by antiresorptive treatment [15]. In a clinical phase III trial, 12 months treatment with romosozumab, followed by alendronate, significantly reduced the risk of vertebral and non-vertebral including hip fractures versus alendronate alone in postmenopausal women with severe osteoporosis [16].
The effective bone-forming action of romosozumab may offer an opportunity to achieve an improved fracture risk reduction among patients with a recent fracture compared with previously available treatment options. Starting bone-building treatment with romosozumab immediately after the fracture event and following up with antiresorptive treatment improves bone mineral density (BMD) significantly. Sequential therapy could thereby more effectively prevent new fractures, as compared with traditional treatment pathway where patients start antiresorptive treatment following the fracture event, or starting bone-building treatment without a subsequent antiresorptive [17]. With recent Swedish treatment guidelines recommending subsequent antiresorptive treatment after romosozumab [18], and romosozumab having been evaluated in its pivotal trial ARCH as a sequential treatment [16], the cost-effectiveness of romosozumab should be assessed assuming that it is used in a treatment sequence.
The objective of this study was to assess the cost-effectiveness of sequential romosozumab followed by alendronate (“romosozumab-to-alendronate”) compared with the use of alendronate alone from a Swedish societal perspective using a Markov microsimulation model for the treatment of postmenopausal women with severe osteoporosis at high risk of fracture.
Methods
Target patient population
In line with the approved indication for romosozumab, the target patient population for the analysis consisted of Swedish women with severe osteoporosis at high risk of fracture. The base case population used in the model was selected to be similar to the average population within the approved indication. Age at treatment start was assumed to be 74 years which was the mean age in the phase III clinical trial ARCH [16]. Femoral neck BMD T-score was assumed to be − 2.5, and all patients were assumed to have a recent MOF; including hip, vertebral, forearm, or proximal humerus).
Model structure
A Markov microsimulation model was used to estimate the cost-effectiveness of romosozumab-to-alendronate compared with alendronate alone. In a microsimulation model, patients are evaluated individually and a record of previous health states for each patient is created within the model. The patient is tracked through health states for which costs and benefits are accumulated over time. These models are appropriate when many health states are of relevance as well as when patients are assumed to be at a changing risk of incurring multiple events with long term consequences, as is the case with osteoporosis. The model is described in more detail in Söreskog et al. (draft manuscript) [19].
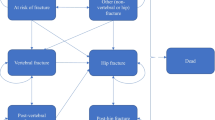
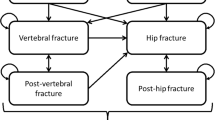
The model consisted of five health states; ‘At risk of fracture’, ‘Hip fracture’, ‘Vertebral fracture’, ‘Non-hip-non-vertebral fracture (NHNV)’, and ‘Death’. The model cycle length was 6 months, which is commonly used in cost-effectiveness models for osteoporosis treatments [20]. In the model, all patients started in the ‘At risk’ health state, and at the end of each 6-month cycle, they had a probability of incurring new hip, vertebral, or NHNV fractures, remaining in a health state without a new fracture, or dying. In case of death, the patient transitioned to the ‘Death’ state and stayed there during the remainder of the simulated time horizon and incurred no further events or costs (absorbing health state). Figure 1 summarizes the health states and possible transitions.
Due to the chronic nature of osteoporosis, a lifetime horizon was chosen in the base-case analysis. All patients were followed on an individual basis from their age at start of treatment until the age of 100 years or death, whichever came first.
Treatment duration and persistence
In the economic analyses, patients in the romosozumab-to-alendronate treatment arm were assumed to be treated for a maximum of 1 year with romosozumab immediately followed by 4 years with alendronate, while patients in the alendronate alone treatment arm were assumed to remain on treatment with alendronate for a maximum of 5 years consistent with Swedish treatment guidelines that recommend re-evaluation of treatment after 5 year [18]. Only patients who were persistent with romosozumab for the initial 1 year were switched to alendronate.
Persistence with romosozumab and sequential alendronate in clinical practice is unknown due to its recent approval. Treatment completion rates in phase III clinical trial ARCH were ~ 90% at 12 months with romosozumab and ~ 77% at primary analysis with sequential alendronate [21]. Romosozumab persistence for this economic evaluation was conservatively assumed to be 80% at 12 months. The proportion persistent to teriparatide, an injectable bone-forming agent administered daily, has been estimated to 70% after 1 year [22]. Persistence with romosozumab was assumed to be higher than teriparatide since less frequently administered drugs have been shown to be associated with greater persistence [23]. Persistence for each time point and treatment is shown in Supplementary Table 5.
Fracture risk and efficacy
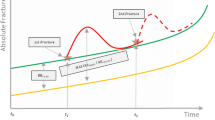
The fracture risk in the model was based on a composite of three elements: the general population risk of incurring a fracture, the increased risk of fracture associated with severe osteoporosis (the relative risk) compared to the general population, and the potential risk reduction attributed to a treatment (versus placebo). Risk was updated every time a patient sustained a fracture. The relative risk of fracture was estimated using the FRAX tool combined with Swedish data on the impact of imminent fracture risk [24]. FRAX was chosen as it is recommended to use to estimate fracture risk in many treatment guidelines [25]. Algorithms based on Swedish retrospective data were used to estimate time-dependent (divided in 6-month intervals) relative risk in patients. At treatment start, it was assumed that fracture risk corresponded to a person with a MOF in the last 6 months. Figure 2 provides an example of how fracture risk is estimated in patients with a first fracture. When the patient sustained their first fracture, risk corresponds to the normal populations risk adjusted for clinical risk factors according to FRAX,Footnote 1 and the maximum of the time-dependent first recent fracture relative risk (RR) and RR of fracture history according to FRAX. In the cost-effectiveness analysis, this corresponds to the time point when treatment is assumed to commence.
Efficacy of romosozumab-to-alendronate vs. alendronate alone on the risk of hip, vertebral and NHNV fractures were sourced from the phase III trial ARCH that studied the efficacy of romosozumab followed by alendronate compared with alendronate alone [16]. To calculate hazard ratios for romosozumab-to-alendronate vs. placebo, the hazard ratios of romosozumab-to-alendronate vs. alendronate alone were multiplied with hazard ratios of alendronate vs. placebo based on a network meta-analysis (NMA). Hazard ratios were differentiated by 6-month time period. In the NMA, it was not possible to extract non-cumulative effect from the non-romosozumab trials due to limited data available in the publications. The cumulative effect of 0–12 and 0–24 months for alendronate based on the NMA was used in the model for the first year and following years, respectively. Hazard ratios for fracture occurrences are included in the model are described in Table 1.
Anti-fracture efficacy persists for a time (offset time) following treatment discontinuation but few studies have directly evaluated the duration of offset after stopping treatment [21, 26,27,28]. The results of those studies vary but indicate that the residual efficacy may persist for at least as long as the time on treatment (except for denosumab that likely has a shorter offset duration). In line with previous research, following treatment discontinuation, treatment efficacy was assumed to linearly decline to zero over a period corresponding to the time a patient remained on treatment.
Epidemiological and cost data
Epidemiological and economic data representative of the Swedish population were used to populate the economic model. The incidence of fracture in the general population was derived from a prospective study in Malmö, Sweden [4, 29]. The age- and sex-adjusted all-cause mortality rates for the general population in Sweden and year 2018 were sourced from Statistics Sweden life tables [30]. Time-dependent increase in fracture risk following the first, second, and third fracture was based on analyses of Swedish register data [24].
First-year costs of hip, vertebral, and NHNV fractures were taken from a study by Borgström et al. of women with osteoporosis in Sweden which are the most recent data appropriate for use in a health economic model [31]. Non-institutional care costs of hip and vertebral fractures second and subsequent years were sourced from an assessment of bisphosphonates by the National Institute of Care Excellence in the UK due to lack of Swedish estimates [32]. Probability of discharge to institutional care after hip fracture (4–34%, depending on age) was based on a study of Nanjayan et al. [33]. The daily cost of long-term care used in the model was assumed to be €155 based on a study by Hernlund et al. [1]. Resource utilization, the corresponding unit costs, and sources are described in Supplementary Table 2. Alendronate drug price was sourced from the Swedish Dental and Pharmaceutical Benefits Agency database [34]. A 3.0% discount factor was applied on costs and health effects in line with Swedish health technology assessment guidelines [35]. All costs are stated in Euro (€) 2019 prices.
The impact on quality of life during the first and subsequent years after hip, vertebral, and NHNV fractures was based on the data from the International Costs and Utilities Related to Osteoporotic Fractures Study (ICUROS) [36]. The multipliers (Supplementary Table 3) were used together with population tariff values for Sweden (Supplementary Table 4) [37].
Analysis
The main outcome of this economic evaluation was the incremental cost-effectiveness ratio (ICER) representing the additional costs required to gain one additional QALY with romosozumab followed by alendronate against alendronate alone. Deterministic sensitivity analyses (DSA) were conducted to estimate the impact on the ICER of changing one parameter input at a time. The impact of changing time horizon, persistence rate, treatment efficacy, treatment start age discount rate, and maximum treatment duration were tested. Probabilistic sensitivity analyses (PSA) were conducted by simultaneous sampling from estimated probability distributions of model parameters to obtain 1000 sets of model input estimates. Distributional assumptions for the model parameters were as follows: the unit costs of drugs were taken as given and not sampled in the model. All other cost parameters were sampled assuming a lognormal distribution since costs are left-skewed and a standard error of 25% of the base-case value. The utility multipliers for hip, vertebral, and NHNV fractures, respectively, were sampled using a lognormal distribution with standard errors based on study data described above. Persistence to treatment and the proportion of patients admitted into long-term care after a hip fracture were sampled assuming beta distributions. A hypothetical societal willingness-to-pay (WTP) per QALY gained threshold of €80,000 was applied, which is a commonly referenced threshold value in Sweden [38]. As a sensitivity analysis, a WTP of €60,000 per QALY gained was tested.
Results
Base case
The results from the base case analysis are presented in Table 2. A patient treated with romosozumab-to-alendronate was expected to accrue 8.547 QALYs and a cost of €60,396. The corresponding result for alendronate alone was 8.458 QALYs at a cost of €57,394. In incremental terms, romosozumab-to-alendronate was associated with 0.089 additional QALYs at an additional cost of €3002 compared to alendronate alone, resulting in an ICER of €33,732.
Sensitivity analyses
Deterministic sensitivity analysis (DSA)
The results from the DSA are provided in Table 3. The ICER was most sensitive to time horizon, persistence rate, and efficacy of alendronate on hip fractures. Romosozumab-to-alendronate was demonstrated to be cost saving (decreases cost and increases QALYs) compared with alendronate alone when assuming efficacy of alendronate on hip fractures to the upper 95% confidence interval. Lower treatment persistence with romosozumab-to-alendronate decreased cost-effectiveness against alendronate alone. Cost-effectiveness is impacted by age at treatment start. The ICER was above €90,000 at age 50 and 60 years, and below €50,000 at age 70 and 80 years. When including patients with BMD T-score of − 2.5 or less, the ICER decreases to €31,152.
Probabilistic sensitivity analysis (PSA)
The PSA shows that the base case results were robust to uncertainty in the input parameters (Table 4). A probabilistic incremental cost of €2676 and corresponding incremental QALYs of 0.094 was estimated, resulting in a probabilistic ICER of €28,467 (base case €33,732). The resulting cost-effectiveness acceptability curves (CEAC) are depicted in Fig. 3. The probability that romosozumab-to-alendronate is cost-effective vs. alendronate alone is 97.9% at a WTP for a QALY threshold of €60,000 and 99.7% at a WTP of €80,000.
Discussion
The objective of this study was to assess the cost-effectiveness of sequential romosozumab-to-alendronate against alendronate alone for the treatment of postmenopausal women with severe osteoporosis at high risk of fracture. The analysis was conducted using a Markov micro simulation model with a structure similar to several previously published models for osteoporosis interventions [20]. The model incorporated two new features that have not been included in previous models for osteoporosis treatments: treatment sequencing and the time-dependent risk contribution from recent fractures. The main finding of the study is that sequential treatment with romosozumab-to-alendronate is expected to increase QALYs and costs, compared with alendronate alone. The incremental cost-effectiveness ratio was €33,732 per QALY in the base case analysis which is below the hypothetical WTP for a QALY of €80,000. In practice, there is no set WTP threshold for pharmaceutical interventions in Sweden, but the WTP is linked to an assessment of disease severity among other factors. Osteoporosis in adults at high risk of fracture has been assessed to have a ‘medium high’ level of severity by the Swedish Dental and Pharmaceutical Benefits Agency (TLV) [39]. In a study of TLV decisions during 2005–2011, the median accepted ICER for severe diseases (excluding cancer) was SEK363,000 (approximately €34,300) [40]. This indicates that romosozumab-to-alendronate can be considered cost-effective for the treatment of postmenopausal women with severe osteoporosis at high risk of fracture in Sweden.
The cost-effectiveness ratio estimated in our study can be compared to that of other treatment evaluations in Swedish setting. However, there are few published studies that compare two active treatments with each other like in this analysis. One analysis assessed the cost-effectiveness for denosumab compared with generic alendronate and the cost per QALY was estimated at €27,000 in the year 2010 [20]. There have also been a few publications on the cost-effectiveness of other bone-forming agents, mainly teriparatide. For example, a study compared teriparatide compared with no treatment, yielding an ICER of €43,473 [41]. However, since this study did not compare against alendronate and did not consider treatment sequencing a comparison with this study is not meaningful to assess the external validity of our estimates.
Sensitivity analyses show that the results were robust when varying diverse input parameters in the model; the ICER was most sensitive to changes to the time horizon, treatment persistence, and efficacy of alendronate on hip fractures. The confidence interval around the hazard ratio at 12 months of hip fracture for alendronate was wide (hazard ratio 0.72, 95% confidence interval 0.12–2.38), leading to romosozumab-to-alendronate dominating alendronate when assuming the upper bound of this interval. The ICER of romosozumab followed by alendronate versus alendronate alone remained below the hypothetical Swedish WTP threshold of €80,000 in all sensitivity analyses. Based on the sensitivity analyses, romosozumab-to-alendronate was less likely to be cost-effective at ages below 60 years. This arises because of lower fracture risks in the younger populations, despite having a recent fracture and low BMD T-score. In clinical practice, it is expected that the proportion of the target patient population within the young groups is small since fracture risks increase exponentially with age, and the mean age in patient initiating osteoporosis treatment is around 72 years [23].
Real-world persistence data with romosozumab-to-alendronate is lacking. Teriparatide, another bone-forming agent used for the treatment of postmenopausal osteoporosis, has a 1-year persistence of approximately 70% [22]. Studies indicate that less frequent administration (e.g. daily vs weekly) is associated with better persistence [42]. Given that romosozumab is intended to be administered less frequently than teriparatide (monthly vs. daily), it is reasonable to assume that patients treated with romosozumab will have a better persistence compared with teriparatide. The magnitude is unknown; however, it was conservatively assumed that 80% of patients will remain on treatment with romosozumab throughout its 12-month treatment length. Alendronate, orally administered daily or weekly, is known to have poor persistence. About 50% discontinue treatment within 1 year [22]. For the alendronate sequence following romosozumab, it was assumed that patients will have a persistence corresponding to the real-world persistence on alendronate 1 year into treatment with alendronate. In the future, when real-world persistence data with romosozumab-to-alendronate come available, the cost-effectiveness analyses may be updated.
In this study, alendronate was chosen as the main comparator and the sequential treatment for romosozumab, as alendronate represents the most commonly used treatment in the relevant patient population. Further, alendronate was the comparator in the only phase III study of romosozumab made in a patient population where all had prior fracture [16]. In clinical practice, however, it is possible that some patients may receive zoledronate or denosumab following romosozumab.
In the model, treatment was assumed to start within 6 months after an initial MOF. However, in reality, time to treatment after fracture, in those who actually receive treatment, may be longer than 12 months. In a study based on Swedish register data from 2007 to 2011, the proportion of patients with hip fracture starting treatment within 3 and 6 months (out of those starting within 12 months) was 40% and 70%, respectively [13]. Thus about 30% of those starting treatment within 12 months are expected in month 7–12. There is, however, a possibility that with the increased awareness of imminent fracture risk after fracture, as well as increased implementation of fracture liaison services, a larger proportion of patients would be captured closer to the time of the initial fracture.
Simplification is necessary in cost-effectiveness modelling. Consequently, uncertainty is introduced, primarily related to assumptions and model inputs. Validation of the model by clinical experts was undertaken to reduce uncertainty. However, some key inputs remain uncertain, and some simplifications cannot be avoided. Fracture efficacy data of alendronate were only available for the time periods 0–12 months and 24 months. It was therefore not possible to specify efficacy in more granular time intervals as for romosozumab (6 months intervals). However, alendronate could be expected to have a slower onset than romosozumab, and the available granularity may be sufficient to capture alendronate’s efficacy over time. Another uncertainty relates to the general population fracture incidences used in the model which were based on fracture recordings from 1987 to 1994 [4]. More recent data would have been desirable; however, this has not been sufficiently published.
Recently, probability ratios have been developed to adjust conventional estimates of FRAX according to the recency of a sentinel fracture derived from a large population-based cohort in Iceland [17, 43]. For example, for a woman at age 70 years, a prior clinical vertebral fracture within the past 2 years is associated with a 1.5-fold higher fracture probability than for a woman of the same age with a prior fragility fracture of uncertain recency. In the context of FRAX for Sweden, the 10-year probability of a MOF is uplifted from 25 to 38%. Hip fracture probability is uplifted from 8.8 to 12% for the same clinical scenario. However, probably adjustment ratios were very sensitive to age (as well as to recency of fracture and the site of prior fracture). In the case of age and sentinel vertebral fracture, adjustment ratios varied from 7.1 at the age of 40 years to 0.9 at the age of 90 years, respectively. The adjustment ratios used in the model were based on Swedish register data. These ratios were adjusted for several covariates; however, not all clinical risk factors were available in the register data. This entails that the modelled fracture risk may to some extent be overestimated; however, the impact is likely limited. In future developments of the model, the new adjustment ratios incorporated in FRAX will be included in the model to improve the prediction of fracture risk.
A strength of this study was the incorporation of time-dependent fracture risk in patients with recent fracture. This feature has not been available in previous cost-effectiveness models of osteoporosis treatments. In prior models, the risk contribution of a historical fracture has been assumed to be constant over time possibly underestimating the imminent fracture risk. Further, these estimates were based on Swedish retrospective real-world data, which better reflects patients whom may be eligible for treatment in Swedish clinical practice. Another novel feature of this study was the incorporation of treatment sequences, which better reflects the treatment regimen for bone-forming agents and the chronic treatment as signalled by guidelines and label indication. An underlying assumption of this analysis is to evaluate romosozumab as treatment sequence to reflect its pivotal trial and Swedish treatment guidelines [16, 18]. Evaluating romosozumab as a non-sequential treatment underestimates its cost-effectiveness, as recently demonstrated by Söreskog et al. [manuscript submitted] [19].
Conclusion
The results indicate that sequential treatment with romosozumab followed by alendronate compared to alendronate alone can be a cost-effective treatment option for postmenopausal women with osteoporosis and a recent fracture in Sweden.
Data availability
Not applicable.
Notes
Smoking, secondary osteoporosis, body mass index, femoral neck T-score, parental hip fracture, rheumatoid arthritis, glucocorticoid use corresponding to ≥ 5 mg prednisolone/day for more than 3 months, alcohol (≥ 3 units/day), and secondary osteoporosis
References
Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8:136
Svedbom A, Hernlund E, Ivergård M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA (2013) Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos 8:137
Kanis JA, Oden A, McCloskey EV, Johansson H, Wahl DA, Cooper C, Epidemiology, IOFWGo, and Quality of, L (2012) A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int 23(9):2239–2256
Kanis JA, Johnell O, Oden A, Sembo I, Redlund-Johnell I, Dawson A, De Laet C, Jonsson B (2000) Long-term risk of osteoporotic fracture in Malmo. Osteoporos Int 11(8):669–674
Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, Eisman J, Fujiwara S, Garnero P, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35(2):375–382
Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA 3rd, Berger M (2000) Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 15(4):721–739
Banefelt J, Åkesson KE, Spångéus A, Ljunggren O, Karlsson L, Ström O, Ortsäter G, Libanati C, Toth E (2019) Risk of imminent fracture following a previous fracture in a Swedish database study. Osteoporos Int 30(3):601–609
Bliuc D, Nguyen ND, Nguyen TV, Eisman JA, Center JR (2013) Compound risk of high mortality following osteoporotic fracture and refracture in elderly women and men. J Bone Miner Res 28(11):2317–2324
Johansson H, Siggeirsdottir K, Harvey NC, Oden A, Gudnason V, McCloskey E, Sigurdsson G, Kanis JA (2017) Imminent risk of fracture after fracture. Osteoporos Int 28(3):775–780
van Geel TA, van Helden S, Geusens PP, Winkens B, Dinant GJ (2009) Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis 68(1):99–102
Akesson K, Marsh D, Mitchell PJ, McLellan AR, Stenmark J, Pierroz DD, Kyer C, Cooper C, Group, IOFFW (2013) Capture the fracture: a best practice framework and global campaign to break the fragility fracture cycle. Osteoporos Int 24(8):2135–2152
Harvey NC, McCloskey EV, Mitchell PJ, Dawson-Hughes B, Pierroz DD, Reginster JY, Rizzoli R, Cooper C, Kanis JA (2017) Mind the (treatment) gap: a global perspective on current and future strategies for prevention of fragility fractures. Osteoporos Int 28(5):1507–1529
Jonsson E, Eriksson D, Akesson K, Ljunggren O, Salomonsson S, Borgstrom F, Strom O (2015) Swedish osteoporosis care. Arch Osteoporos 10:222
European Medicines Agency (2020) Evenity (romosozumab)
EVENITY (romosozumab) Summary of Product Characteristics
Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A (2017) Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 377(15):1417–1427
Kanis JA, Harvey NC, McCloskey E, Bruyere O, Veronese N, Lorentzon M, Cooper C, Rizzoli R, Adib G, Al-Daghri N, Campusano C, Chandran M, Dawson-Hughes B, Javaid K, Jiwa F, Johansson H, Lee JK, Liu E, Messina D, Mkinsi O, Pinto D, Prieto-Alhambra D, Saag K, Xia W, Zakraoui L, Reginster J (2020) Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int 31(1):1–12
Swedish Medical Products Agency (Läkemedelsverket) (2020) Pharmaceuticals for osteoporosis to prevent fragility fractures– treatment recommendation ["Läkemedel vid osteoporos för att förhindra benskörhetsfrakturer– behandlingsrekommendation"]
Söreskog, S, Borgström, F, Lindberg, I, Ström, O, Willems, D, Libanati, C, Kanis, JA, Stollenwerk, B, Charokopou, M (2020) A novel economic framework to assess the cost-effectiveness of bone-forming agents in the prevention of fractures in patients with osteoporosis. Manuscript submitted
Jonsson B, Strom O, Eisman JA, Papaioannou A, Siris ES, Tosteson A, Kanis JA (2011) Cost-effectiveness of Denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int 22(3):967–982
Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR, Group, FR (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the fracture intervention trial long-term extension (FLEX): a randomized trial. JAMA 296(24):2927–2938
Landfeldt E, Strom O, Robbins S, Borgstrom F (2012) Adherence to treatment of primary osteoporosis and its association to fractures--the Swedish adherence register analysis (SARA). Osteoporos Int 23(2):433–443
Jonsson E, Eriksson D, Åkesson K, Ljunggren Ö, Salomonsson S, Borgström F, Ström O (2015) Swedish osteoporosis care. Arch Osteoporos 10(1):24
Soreskog E, Strom O, Spangeus A, Akesson KE, Borgstrom F, Banefelt J, Toth E, Libanati C, Charokopou M (2020) Risk of major osteoporotic fracture after first, second and third fracture in Swedish women aged 50years and older. Bone 134:115286
Kanis JA, Harvey NC, Cooper C, Johansson H, Oden A, McCloskey EV, Advisory Board of the National Osteoporosis Guideline, G (2016) A systematic review of intervention thresholds based on FRAX : a report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch Osteoporos 11(1):25
Black DM, Reid IR, Boonen S, Bucci-Rechtweg C, Cauley JA, Cosman F, Cummings SR, Hue TF, Lippuner K, Lakatos P, Leung PC, Man Z, Martinez RL, Tan M, Ruzycky ME, Su G, Eastell R (2012) The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 27(2):243–254
Lindsay R, Scheele WH, Neer R, Pohl G, Adami S, Mautalen C, Reginster JY, Stepan JJ, Myers SL, Mitlak BH (2004) Sustained vertebral fracture risk reduction after withdrawal of teriparatide in postmenopausal women with osteoporosis. Arch Intern Med 164(18):2024–2030
Prince R, Sipos A, Hossain A, Syversen U, Ish-Shalom S, Marcinowska E, Halse J, Lindsay R, Dalsky GP, Mitlak BH (2005) Sustained nonvertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J Bone Miner Res 20(9):1507–1513
Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A (2001) The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 12(5):417–427
Statistics Sweden (2018) Ettårig livslängdstabell, dödsrisker (promille), efter kön, ålder och år
Borgström F, Zethraeus N, Johnell O, Lidgren L, Ponzer S, Svensson O, Abdon P, Ornstein E, Lunsjö K, Thorngren KG (2006) Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int 17(5):637–650
Davis Sea (2015) Bisphosphonates for preventing osteoporotic fragility fractures (including a partial update of NICE technology appraisal guidance 160 and 161). Assessment report. Table 32., National Institute for Health and Care Excellence (NICE) (ed)
Nanjayan SK, John J, Swamy G, Mitsiou K, Tambe A, Abuzakuk T (2014) Predictors of change in 'discharge destination' following treatment for fracture neck of femur. Injury 45(7):1080–1084
The Dental and Pharmaceutical Benefits Agency (TLV) (2019) Price database Alendronate. 2019-12-10]
The Dental and Pharmaceutical Benefits Agency (TLV) (2017) Ändring i Tandvårds- och läkemedelsförmånsverkets allmänna råd (TLVAR 2003:2) om ekonomiska utvärderingar
Kanis JA, Johansson H, Oden A, Harvey NC, Gudnason V, Sanders KM, Sigurdsson G, Siggeirsdottir K, Fitzpatrick LA, Borgstrom F, McCloskey EV (2018) Characteristics of recurrent fractures. Osteoporos Int 29(8):1747–1757
Burström K, Johannesson M, Diderichsen F (2001) Swedish population health-related quality of life results using the EQ-5D. Qual Life Res 10(7):621–635
The Dental and Pharmaceutical Benefits Agency (TLV) Kostnadseffektiva läkemedel (Cost-effective pharmaceuticals). 2020-06-23]; Available from: https://www.tlv.se/download/18.467926b615d084471ac3396c/1510316400262/kostnadseffektiva-lakemedel.pdf
The Dental and Pharmaceutical Benefits Agency (TLV) (2014) Reimbursement decision Prolia. of Medium] Last Update [cited Access 2014; Available from: https://www.tlv.se/download/18.467926b615d084471ac3342b/1510316384854/bes141120-prolia.pdf
Svensson, M and Nilsson, FJL(2016) TLV: s betalningsvilja för nya läkemedel har analyserats: Kostnadseffektivitet och sjukdomens svårighetsgrad avgörande för subvention-Cancerläkemedel får kosta mer. 113(28–30)
Borgstrom F, Strom O, Marin F, Kutahov A, Ljunggren O (2010) Cost effectiveness of teriparatide and PTH(1-84) in the treatment of postmenopausal osteoporosis. J Med Econ 13(3):381–392
Karlsson L, Lundkvist J, Psachoulia E, Intorcia M, Strom O (2015) Persistence with denosumab and persistence with oral bisphosphonates for the treatment of postmenopausal osteoporosis: a retrospective, observational study, and a meta-analysis. Osteoporos Int 26(10):2401–2411
Kanis JA, Johansson H, Harvey NC, Gudnason V, Sigurdsson G, Siggeirsdottir K, Lorentzon M, Liu E, Vandenput L, McCloskey EV (2020) Adjusting conventional FRAX estimates of fracture probability according to the recency of sentinel fractures. Osteoporos Int 31(10):1817–1828
Funding
This study was funded by UCB Pharma and Amgen.
Author information
Authors and Affiliations
Contributions
All authors participated in designing the study. ES, IL, and FB performed the analyses and drafted the manuscript. DW, ML, JK, OS, and PB provided intellectual input on the manuscript. JK and ML provided clinical expertise. All authors interpreted the results and provided feedback. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
ES, IL, and FB are employees of Quantify Research which was contracted and paid by UCB Pharma to conduct the study. The authors did not receive direct payment as a result of this work outside of their normal salary payments. PB and DW are employees of UCB Pharma. JAK reports grants from Amgen, Eli Lilly, and Radius Health; consulting fees from Theramex. JAK is the architect of FRAX® but has no financial interest. KEA reports lecture and advisory fees outside this work from Amgen, Astellas, Chugai, Renapharma, and UCB Pharma. ML received lecture fees outside this work from Amgen, Astellas, Eli Lilly, UCB Pharma, Radius Health, Meda, GE-Lunar, and Santax Medico/Hologic.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 37 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Söreskog, E., Lindberg, I., Kanis, J. et al. Cost-effectiveness of romosozumab for the treatment of postmenopausal women with severe osteoporosis at high risk of fracture in Sweden. Osteoporos Int 32, 585–594 (2021). https://doi.org/10.1007/s00198-020-05780-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05780-8