Abstract
Introduction and hypothesis
Bladder pain syndrome (BPS) is characterised by chronic pain in the bladder area accompanied by urgency and/or frequency without the presence of other confusable diseases. Owing to a lack of gold standard diagnostic tests and definitive cure it is paramount to define treatment goals and validated measurements of outcomes. Patient-reported outcome measures (PROMs) are validated questionnaires completed by patients that can help to reduce ambiguity in the BPS patient treatment pathway, but they are currently underutilised. We present to our knowledge the first summary and analysis of all available PROMs in BPS patients.
Methods
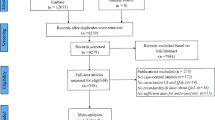
Review and critical evaluation of all relevant BPS guidelines presented in English language and a systematic search for PubMed database articles relating to PROMs and subjective assessment grading tools in BPS, interstitial cystitis and chronic pelvic pain syndrome.
Results
The ideal PROMs for BPS should assess urinary symptoms, pain, quality of life and sexual health. There are five PROMs designed specifically for BPS patients. The most universally used and quoted is the O’Leary–Sant questionnaire followed by the Pelvic Pain and Urgency Score and the Wisconsin Interstitial Cystitis scale. However, there is no single PROM for BPS that is ideal, and for comprehensive assessment several questionnaires are often used simultaneously.
Conclusions
Patient-reported outcome measures are a valuable tool for use in the long-term management of patients burdened with BPS. There are now several disease-specific PROMs in use that have their respective advantages and disadvantages. Their use should be encouraged in future research as well as continued efforts to develop new PROMs that can address current shortcomings.
Similar content being viewed by others
Change history
12 July 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00192-023-05605-2
References
Malde S, Palmisani S, Al-Kaisy A, Sahai A. Guideline of guidelines: bladder pain syndrome. BJU Int. 2018;122:729–43. https://doi.org/10.1111/bju.14399.
Engler DS, Baranowski AP. EAU Guidelines on “Chronic Pelvic Pain”. EAU Guidelines Office, Arnhem, The Netherlands. Edn. presented at the EAU Annual Congress Amsterdam 2022.
Juliebø-Jones P, Hjelle KM, Mohn J, et al. Management of bladder pain syndrome (BPS): a practical guide. Adv Urol. 2022;2022:1–9. https://doi.org/10.1155/2022/7149467.
Jones P, Hjelle KM, Mohn J, et al. Current Status of Intravesical Therapies for Bladder Pain Syndrome (BPS): A Narrative Review of Emerging Evidence. Urol. 2021;156:e48–57. https://doi.org/10.1016/j.urology.2021.05.042.
Wu A, Snyder C. Getting ready for patient-reported outcomes measures (PROMs) in clinical practice. Healthc Pap. 2012;11:48–53. https://doi.org/10.12927/hcpap.2012.22705.
Kluzek S, Dean B, Wartolowska KA. Patient-reported outcome measures (PROMs) as proof of treatment efficacy. BMJ Evid Based Med. 2022;27:153–5. https://doi.org/10.1136/bmjebm-2020-111573.
Mehmi A, Jones P, Somani BK. Current status and role of patient-reported outcome measures (PROMs) in endourology. Urology. 2021;148:26–31. https://doi.org/10.1016/j.urology.2020.09.022.
Ratti MM, Gandaglia G, Alleva E, et al. Standardising the assessment of patient-reported outcome measures in localised prostate cancer. Syst Rev Eur Urol Oncol. 2022;5:153–63. https://doi.org/10.1016/j.euo.2021.10.004.
Narang GL, Pannell SC, Laviana AA, et al. Patient-reported outcome measures in urology. Curr Opin Urol. 2017;27:366–74. https://doi.org/10.1097/MOU.0000000000000412.
Vasudevan V, Moldwin R. Addressing quality of life in the patient with interstitial cystitis/bladder pain syndrome. Asian J Urol. 2017;4:50–4. https://doi.org/10.1016/j.ajur.2016.08.014.
Bogart LM, Suttorp MJ, Elliott MN, Clemens JQ, Berry SH. Prevalence and correlates of sexual dysfunction among women with bladder pain syndrome/interstitial cystitis. Urology. 2011;77:576–80. https://doi.org/10.1016/j.urology.2010.10.016.
Keller ML, McCarthy DO, Neider RS. Measurement of symptoms of interstitial cystitis. A pilot study. Urol Clin North Am. 1994;21:67–71.
Porru D, Tinelli C, Gerardini M, Giliberto GL, Stancati S, Rovereto B. Evaluation of urinary and general symptoms and correlation with other clinical parameters in interstitial cystitis patients. Neurourol Urodyn. 2005;24:69–73. https://doi.org/10.1002/nau.20084.
Propert KJ, Mayer RD, Wang Y, et al. Responsiveness of symptom scales for interstitial cystitis. Urology. 2006;67:55–9. https://doi.org/10.1016/j.urology.2005.07.014.
Erickson DR, Morgan KC. Nonbladder related symptoms in patients with interstitial cystitis. J Urol. 2001;166:557–62.
Goin JE, Olaleye D, Peters KM, Steinert B, Habicht K, Wynant G. Psychometric analysis of the University of Wisconsin Interstitial Cystitis Scale: implications for use in randomized clinical trials. J Urol. 1998;159:1085–90. https://doi.org/10.1016/S0022-5347(01)63840-0.
O’Leary MP, Sant GR, Fowler FJ, Whitmore KE, Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997;49:58–63. https://doi.org/10.1016/S0090-4295(99)80333-1.
Lubeck DP, Whitmore K, Sant GR, Alvarez-Horine S, Lai C. Psychometric validation of the O’Leary-Sant interstitial cystitis symptom index in a clinical trial of pentosan polysulfate sodium. Urol. 2001;57:62–6. https://doi.org/10.1016/S0090-4295(01)01126-8.
Kuo Y-C, Kuo H-C. O’Leary-Sant Symptom Index predicts the treatment outcome for onabotulinumtoxinA injections for refractory interstitial cystitis/bladder pain syndrome. Toxins (Basel). 2015;7:2860–71. https://doi.org/10.3390/toxins7082860.
Huang M-C, Hsieh C-H, Chang W-C, Chang S-T, Lee M-S. Assessment of treatment outcomes of interstitial cystitis with hydrodistention and bladder training by O’Leary-Sant Interstitial Cystitis Symptom and Problem Indices. Taiwan J Obstet Gynecol. 2018;57:718–21. https://doi.org/10.1016/j.tjog.2018.08.019.
Cvach K, Rosamilia A, Dwyer P, et al. Efficacy of clorpactin in refractory bladder pain syndrome/interstitial cystitis: a randomized controlled trial. Int Urogynecol J. 2021;32:1177–83. https://doi.org/10.1007/s00192-020-04652-3.
Kabay S, Kabay S, Sevim M. First-line treatment posterior tibial nerve stimulation in patients with interstitial cystitis/bladder pain syndrome. Cent European J Urol 2021;74:208–14. https://doi.org/10.5173/ceju.2021.0372.
Nickel JC, Tripp D, Teal V, et al. Sexual function is a determinant of poor quality of life for women with treatment refractory interstitial cystitis. J Urol. 2007;177:1832–6. https://doi.org/10.1016/j.juro.2007.01.060.
Okui N, Okui M, Gambacciani M. Examining vaginal and vulvar health and sexual dysfunction in patients with interstitial cystitis (UNICORN-1 study). Int Urogynecol J. 2022;33:2493–9. https://doi.org/10.1007/s00192-022-05220-7.
Parsons CL, Dell J, Stanford EJ, et al. Increased prevalence of interstitial cystitis: previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology. 2002;60:573–8. https://doi.org/10.1016/S0090-4295(02)01829-0.
Victal ML, D’Ancona CAL, Junqueira RG, Carlos da Silva D, Oliveira HC, de Moraes Lopes MHB. Test-retest reliability and discriminant validity for the Brazilian version of “The Interstitial Cystitis Symptom Index and Problem Index” and “Pelvic Pain and Urgency/Frequency (PUF) Patient Symptom Scale” instruments. Transl Androl Urol. 2015;4:594. https://doi.org/10.3978/J.ISSN.2223-4683.2015.11.01.
Clemens JQ, Calhoun EA, Litwin MS, et al. Validation of a modified National Institutes of Health Chronic Prostatitis Symptom Index to assess genitourinary pain in both men and women. Urology. 2009;74:983–7.e3. https://doi.org/10.1016/j.urology.2009.06.078.
Humphrey L, Arbuckle R, Moldwin R, et al. The Bladder Pain/Interstitial Cystitis Symptom Score: development, validation, and identification of a cut score. Eur Urol. 2012;61:271–9. https://doi.org/10.1016/j.eururo.2011.10.004.
Lorenzo L, Bonillo MA, Arlandis S, et al. Hidrodistensión bajo anestesia más inyección de Onabotulinumtoxin A en pacientes con síndrome de dolor vesical refractario a tratamiento conservador. Actas Urol Esp. 2016;40:303–8. https://doi.org/10.1016/j.acuro.2015.12.011.
Nickel JC, Egerdie B, Davis E, Evans R, Mackenzie L, Shrewsbury SB. A phase II study of the efficacy and safety of the novel oral SHIP1 activator AQX-1125 in subjects with moderate to severe interstitial cystitis/bladder pain syndrome. J Urol. 2016;196:747–54. https://doi.org/10.1016/j.juro.2016.03.003.
Arlandis S, Bonillo MA. [Cystoscopy in the assessment of patients with bladder pain syndrome: results of a national multicenter observational study]. Arch Esp Urol. 2021;74:456–69.
Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4:28–37.
ICIQ website. ICIQ Retrieved from Website: https://www.Iciq.Net/. 2022.
Wilson KA, Dowling AJ, Abdolell M, Tannock IF. Perception of quality of life by patients, partners and treating physicians. Qual Life Res. 2000;9:1041–52. https://doi.org/10.1023/a:1016647407161.
Propert KJ, Payne C, Kusek JW, Nyberg LM. Pitfalls in the design of clinical trials for interstitial cystitis. Urology. 2002;60:742–8. https://doi.org/10.1016/S0090-4295(02)01775-2.
Nickel JC, Barkin J, Forrest J, et al. Randomized, double-blind, dose-ranging study of pentosan polysulfate sodium for interstitial cystitis. Urology. 2005;65:654–8. https://doi.org/10.1016/j.urology.2004.10.071.
Author information
Authors and Affiliations
Contributions
S. Uguzova: project development, manuscript writing; P. Juliebø-Jones: project development, manuscript writing and editing; C. Beisland: manuscript editing, critical revision of the article; A. Haq: manuscript editing, critical revision of the article.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In the original version of this article, the name of the third author was incorrectly spelled as "Christopher Beisland". The correct name is “Christian Beisland”.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uguzova, S., Juliebø-Jones, P., Beisland, C. et al. Current status of patient-reported outcome measures and other subjective assessment grading tools in bladder pain syndrome. Int Urogynecol J 34, 1677–1687 (2023). https://doi.org/10.1007/s00192-023-05551-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-023-05551-z