Abstract
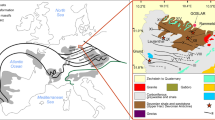
Gold extraction at the Macraes gold mine in New Zealand involves concentration of pyrite and arsenopyrite, oxidation of those sulphides, then cyanidation. The ore concentrate is predominantly Otago Schist host rock (andesitic composition) with up to 15% sulphides. The oxidation step is conducted on ore concentrate slurry in an autoclave at 225°C and 3,800 kPa oxygen gas pressure with continuous feed. The slurry takes ca. 1 h to pass through the autoclave, during which time the sulphides are almost completely oxidised. Sulphide oxidation causes strong acidification of the slurry, which is maintained at pH of 1–2 by addition of CaCO3. Scales form on walls in the autoclave, with minerals reflecting progressive oxidation and alteration of the ore through the system. The schist in the ore feed has mineralogy similar to propylitically altered andesite: quartz, albite, muscovite, chlorite, and pyrite. Muscovite undergoes almost complete dissolution, with associated precipitation of quartz and alunite (KAl3(SO4)2(OH)6). Other principal minerals deposited and discharged include anhydrite (and/or gypsum), jarosite (KFe3(SO4)2(OH)6), hematite (and/or amorphous iron oxyhydroxide), and amorphous arsenates. Dissolved ferrous iron passes right through the autoclave, and variably hydrated Fe2+and Fe3+sulphate minerals, including rozenite and szomolnokite (both FeSO4.hydrate) and ferricopiapite (Fe5(SO4)6O(OH).hydrate), are formed along the way. The autoclave chemical system resembles acid–sulphate hydrothermal activity in geothermal systems and high-sulphidation epithermal mineral deposits formed in arc environments. These natural acid–sulphate systems are pervaded by volcanic vapours in the near-surface environment, where widespread dissolution of host rocks occurs and deposition of quartz, alunite, and anhydrite is common. Some of the volume loss associated with these natural systems may be due to dissolution of soluble sulphate minerals by later-stage groundwater incursion.







Similar content being viewed by others
References
Bethke CM (1998) The geochemist’s workbench, Release 3.0, University of Illinois, USA
Cooke DR, Simmons SF (2000) Characteristics and genesis of epithermal gold deposits. In: Hagemann SG, Brown PE (eds) Gold in 2000. Rev Econ Geol 13:221–244
Craw D (2002) Geochemistry of late metamorphic hydrothermal alteration and graphitisation of host rock, Macraes gold mine, Otago Schist, New Zealand. Chem Geol 191:257–275
Craw D (2003) Geochemical changes in mine tailings during a transition to pressure-oxidation process discharge, Macraes Mine, New Zealand. J Geochem Explor 80:81–94
Fournier RO (1985) Behaviour of silica in hydrothermal systems. In: Berger BR, Bethke PM (eds) Geology and geochemistry of epithermal systems. Rev Econ Geol 2:45–62
Getahun A, Reed MH, Symonds R (1996) Mount St. Augustine volcano fumarole wall rock alteration: mineralogy, zoning, composition and numerical models of its formation process. J Volcan Geotherm Res 71:73–107
Giggenbach WF (1992) Magma degassing and mineral deposition in hydrothermal systems along convergent plate boundaries. Econ Geol 87:1927–1944
Fulignati P, Sbrana A, Luperini W, Greco V (2002) Formation of rock coatings induced by the acid fumarole plume of the passively degassing volcano of La Fossa (Vulcano Island, Italy). J Volcan Geotherm Res 115:397–410
Hedenquist JW, Simmons SF, Giggenbach WF, Eldridge CS (1993) White Island, New Zealand, volcanic-hydrothermal system represents the geochemical environment of high-sulfidation Cu and Au ore deposition. Geology 21:731–734
Hedenquist JW, Izawa E, Arribas A, White NC (1996) Epithermal gold deposits: styles, characteristics, and exploration. Society of Resource Geology Special Publication 1, p 16
Hedenquist JW, Arribas A, Reynolds TJ (1998) Evolution of an intrusion-centered hydrothermal system: far Southeast Lepanto porphyry and epithermal Cu–Au deposits, Phillipines. Econ Geol 93:373–404
Hedenquist JW, Arribas AR, Gonzalez-Urien E (2000) Exploration for epithermal gold deposits. In: Hagemann SG, Brown PE (eds) Gold in 2000. Rev Econ Geol 13:245–277
Henley RW, Ellis AJ (1983) Geothermal systems ancient and modern: a geochemical review. Earth Sci Rev 19:1–50
Johnstone Q, LaBrooy S, Craw D (2001) Formation and remediation of scales, pressure oxidation plant, Macraes Mine, New Zealand. Proceedings Aus Inst Min Metall NZ Branch 34th Ann Conf, pp 275–280
LaBrooy SR, Linge HG, Walker GS (1994). Review of gold-extraction from ores. Miner Eng 7:1213–1241
Mortimer N, Roser BP (1992) Geochemical evidence for the position of the Caples-Torlesse boundary in the Otago Schist, New Zealand. J Geol Soc (Lond) 149:967–977
Papangelakis VG, Demopoulos GP (1991) Acid pressure-oxidation of pyrite: reaction kinetics. Hydrometallurgy 26:309–325
Pokrovski GS, Kara S, Roux J (2002) Stability and solubility of arsenopyrite, FeAsS, in crustal fluids. Geochim Cosmochim Acta 66:2361–2378
Rainbow A, Clark AH, Kyser TK, Gaboury F, Hodgson CJ (2005) The Pierina epithermal Au–Ag deposit, Ancash, Peru: paragenetic relationships, alunite textures, and stable isotope geochemistry. Chem Geol 215:235–252
Reed MH (1997) Hydrothermal alteration and its relationship to ore fluid composition. In: HL Barnes (ed) Geochemistry of hydrothermal ore deposits. Wiley, New York, pp 303–366
Rodgers KA, Hamlin KA, Browne PRL, Campbell KA, Martin R (2000) The steam condensate alteration mineralogy of Ruatapu cave, Orakei Korako geothermal field, Taupo Volcanic Zone, New Zealand. Min Mag (Lond) 64:125–142
Rodgers KA, Cook KL, Browne PRL, Campbell KA (2002) The mineralogy, texture and significance of silica derived from alteration by steam condensate in three New Zealand geothermal fields. Clay Miner 37:299–322
Rye RO, Bethke PM, Wasserman MD (1992) The stable isotope geochemistry of acid sulfate alteration. Econ Geol 87:225–262
Simmons SF, White NC, John DA (2005) Geological characteristics of epithermal precious and base metal deposits. In: Hedenquist, JW, Thompson JFH, Goldfarb RJ, Richards JP (eds) Econ Geol 100th Anniv Vol, pp 485–522
Stoffregen RE (1987) Genesis of acid-sulfate alteration and Au–Cu–Ag mineralization at Summittville, Colorado. Econ Geol 82:1575–1591
White NC, Hedenquist JW (1990) Epithermal environments and styles of mineralization: Variations and their causes, and guidelines for exploration. J Geochem Explor 36:445–474
Whittington BI, Muir D (2000) Pressure acid leaching of nickel laterites: a review. Min Proc Ext Met Rev 21:527–600
Wood CP (1994) Mineralogy at the magma-hydrothermal system interface in andesite volcanoes, New Zealand. Geology 22:75–78
Acknowledgements
Financial support for this study was provided by University of Otago and Oceana Gold (NZ) Ltd. Logistical support for sampling, and frequent useful discussions, were provided by the Oceana staff, particularly Brent Hill, Quenton Johnston, and Steve LaBrooy. Expert laboratory assistance was provided by Damian Walls and Dusk Mains. Helpful comments on the manuscript by Larry Meinert and reviewers David Cooke and James MacDonald resulted in substantial improvement in the presentation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: N. White
Rights and permissions
About this article
Cite this article
Craw, D. Pressure-oxidation autoclave as an analogue for acid–sulphate alteration in epithermal systems. Miner Deposita 41, 357–368 (2006). https://doi.org/10.1007/s00126-006-0064-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00126-006-0064-8