Abstract
Childhood obesity has reached pandemic proportions, and youth-onset type 2 diabetes is following suit. This review summarises the literature on the influence of developmental overnutrition, resulting from maternal diabetes, obesity, maternal dietary intake during pregnancy, excess gestational weight gain, and infant feeding practices, on the aetiology of obesity and type 2 diabetes risk during childhood and adolescence. Key goals of this review are: (1) to summarise evidence to date on consequences of developmental overnutrition; (2) describe shared and distinct biological pathways that may link developmental overnutrition to childhood obesity and youth-onset type 2 diabetes; and (3) to translate current knowledge into clinical and public health strategies that not only target primary prevention in youth, but also encourage primordial prevention during the perinatal period, with the aim of breaking the intergenerational cycle of obesity and diabetes.
Similar content being viewed by others
Introduction
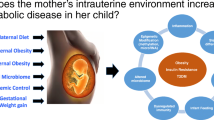
The obesity pandemic has spared no age group, including young children [1]. Following in its footsteps is youth-onset type 2 diabetes, a novel paediatric condition on the rise in the USA [2] and worldwide [3]. The existence and rise in prevalence of paediatric type 2 diabetes is undoubtedly related to trends in childhood obesity given that excess adiposity is the leading risk factor for type 2 diabetes [4, 5] and emerging evidence suggest that both conditions have origins in utero [6,7,8,9]. Little remains known of specific pathways and mechanisms underlying development of youth-onset type 2 diabetes, an important first step to stemming the tide of type 2 diabetes among children and adolescents.
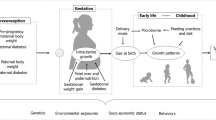
As depicted in Fig. 1, this review expands upon the literature surrounding developmental overnutrition, resulting from maternal diabetes, obesity, diet during pregnancy, and excess gestational weight gain, and infant feeding practices, in relation to childhood obesity and youth-onset type 2 diabetes. We start by summarising and appraising the evidence on consequences of developmental overnutrition and discussing shared and distinct biological pathways that may link developmental overnutrition to obesity and type 2 diabetes in youth. We then translate current knowledge into clinical and public health strategies that not only target primary prevention in youth, but also encourage primordial preventions during the perinatal period, with the aim of breaking the intergenerational cycle of obesity and diabetes [8, 10].
Pathways through which exposure to developmental overnutrition during pregnancy (obesity, maternal diabetes, gestational weight gain, diet during pregnancy, and infant feeding) may influence the development of obesity and type 2 diabetes across the life course. Topics in boxes with a solid border are discussed in depth in this review. This figure is available as a downloadable slide
Developmental overnutrition
In utero overnutrition
Maternal diabetes
Longstanding evidence links maternal diabetes to larger offspring birth size and adiposity across life, and these associations are thought to be driven by maternal fuels: hyperglycaemia and altered lipid and/or amino acid metabolism. While earlier studies evaluated maternal diabetes as a combination of type 1, type 2 and/or gestational diabetes mellitus (GDM), more recent investigations consider diabetes subtypes and degree of hyperglycaemia, which may be more appropriate.
Maternal diabetes, fetal growth and neonatal adiposity
In the 1950s, Pedersen proposed the fuel-mediated teratogenesis hypothesis, which postulated that intrauterine exposure to hyperglycaemia leads to higher birthweight and future obesity and type 2 diabetes risk [6]. This hypothesis is supported by studies showing that women with pre-existing diabetes and those who develop GDM deliver infants with higher birthweight [11,12,13] and fat mass [14, 15]. In a study of 195 women with GDM and 220 control individuals [15], mid-pregnancy fasting glucose was the strongest correlate of newborn fat mass, in comparison with demographic characteristics, family history and maternal anthropometry [15]. Several other studies have since identified associations of maternal hyperglycaemia with offspring adiposity at birth and beyond: (1) maternal glucose levels across all of pregnancy and in the absence of diagnosed diabetes were associated with directly measured neonatal fat mass in the Colorado-based Healthy Start Study (n = 804) [16]; (2) mid-pregnancy oral glucose challenge test glucose levels correlated with higher birthweight among 6854 non-diabetic pregnancies in a study conducted in Texas [17]; (3) higher mid-pregnancy oral glucose tolerance test glucose levels were associated with higher birthweight among >25,000 mother–infant pairs in the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study [18]; and (4) late pregnancy dysglycaemia among non-GDM pregnancies (HbA1c ≥39 mmol/mol at delivery) predicted greater offspring weight gain during early childhood and higher BMI at age 4 years among 898 mother–child pairs in Germany [19]. Together, these findings emphasise the relevance of both degree and timing of maternal hyperglycaemia—even in the absence of frank diabetes—as determinants of offspring adiposity.
Type 1 diabetes, type 2 diabetes and GDM are each associated with altered lipid metabolism [20,21,22], another fuel-mediated pathway through which maternal diabetes may influence newborn adiposity [23]. In the context of GDM, maternal serum NEFA and/or triacylglycerols are associated with higher birthweight [24,25,26]. Findings in the general population have been mixed, with some studies suggesting that the relationship between maternal lipids and neonatal outcomes differs by pre-pregnancy weight status [27,28,29,30]. In the Healthy Heart Study [16] there was a positive association of NEFA during the second half of pregnancy with birthweight independent of pre-pregnancy BMI, but not with newborn fat mass, among 804 mother–infant pairs. There was also a positive relationship between late pregnancy cholesterol and newborn fat mass in overweight/obese women, but not among lean women. Such findings emphasise a need to better understand how specific maternal fuels, singly and in combination with other aspects of maternal health and metabolism, may trigger fuel-mediated overnutrition.
Not surprisingly, given the interplay between lipid and amino acid metabolism [31], altered maternal serum amino acid concentrations have been observed in concomitance with maternal hyperglycaemia. In a study of 67 HAPO participants, targeted metabolomics profiling of maternal serum revealed differences in concentrations of metabolites on amino acid and macronutrient degradation pathways between BMI-matched women with high (>90th percentile) vs low fasting glucose (<10th percentile) at 28 weeks’ gestation [32]. In light of the interrelationships among biochemical mechanisms of nutrient metabolism, future studies interrogating comprehensive metabolite profiles of maternal blood during pregnancy will shed light on cohesive biological pathways linking maternal hyperglycaemia to offspring health.
Maternal diabetes and offspring obesity
The relationship between maternal diabetes and offspring adiposity starts at birth and tracks across the life course. In a survey-based analysis of >14,000 US youth in the Growing Up Today Study (GUTS) [33], maternal GDM correlated with 40% higher odds of being overweight during adolescence (OR 1.4, 95% CI 1.2, 1.6). The estimate was attenuated after accounting for maternal pre-pregnancy BMI (OR 1.2, 95% CI 0.8, 1.7). In the Project Viva pre-birth cohort, among 366 boys aged 6–10 years old, the difference in fat mass between those born to mothers with GDM vs those born to normoglycaemic mothers was attenuated from 2.6 kg (95% CI 1.0, 4.2) to 2.0 kg (95% CI 0.4, 3.6) after adjusting for pre-pregnancy BMI [34]. Similarly, in 461 participants of the Exploring Perinatal Outcomes in CHildren (EPOCH) cohort, GDM exposure correlated with higher BMI, waist circumference, visceral and subcutaneous adipose tissue in offspring at age 6–13 years [35]. The investigators noted modest attenuation (i.e. by 14–42%) in estimates of interest after accounting for pre-pregnancy BMI, with some associations retaining statistical significance [35]. A recent investigation of >5000 youth in The Environmental Determinants of Diabetes in the Young (TEDDY) study reported higher odds of being overweight in 5-year-old offspring exposed to maternal GDM (OR 1.48, 95% CI 1.14, 1.92), type 1 diabetes (OR 1.60, 95% CI 1.16, 2.20) and type 2 diabetes (OR 7.39, 95% CI 2.46, 22.23) compared with their unexposed counterparts [36]; adjustment for maternal pre-pregnancy BMI attenuated all three estimates. Similarly, three meta-analyses reported that adjustment for maternal BMI attenuated, but did not completely abolish, associations of GDM with offspring obesity and abnormal glucose tolerance during childhood [37,38,39]. Taken together, the evidence suggests an independent effect of exposure to diabetes in utero on future adiposity and type 2 diabetes risk.
We note that although inclusion of maternal pre-pregnancy BMI in regression models exploring associations between GDM and offspring adiposity may partly account for genetic predisposition, such adjustment may also control for shared intrauterine mechanisms that lead to fetal overnutrition, since maternal glucose is elevated among overweight/obese women, even if they do not qualify as having diabetes. Use of appropriate analytical approaches (i.e. inverse probability weighting [40] to balance the distribution of pre-pregnancy BMI among women with and without GDM rather than simple adjustment, which may inadvertently block the effect of shared aetiology) and mechanistic studies, will help to ascertain the extent to which accounting for maternal BMI isolates associations of interest.
Maternal diabetes and offspring type 2 diabetes
The longitudinal study of Pima Indians in the Gila River Indian Community [41] was one of the first studies to explore associations of in utero exposure to maternal diabetes (combined pre-existing type 2 diabetes and GDM) with subsequent type 2 diabetes in offspring. In this high-risk population, offspring of women with diabetes not only had higher birthweight [41], but also continued on a trajectory of higher weight-for-height through adolescence [42, 43] and had a tenfold greater risk of developing type 2 diabetes in adolescence and young adulthood [10]. Moreover, exposure to maternal diabetes was the single strongest risk factor for youth-onset type 2 diabetes (OR 10.4, 95% CI 4.3, 25.1) and accounted for most of the dramatic increase in youth-onset type 2 diabetes in this population over the last 30 years [44].
Investigations in lower-risk, racially diverse populations support findings from the Pima Indians. In the SEARCH for Diabetes in Youth Case–Control Study, the odds of type 2 diabetes was 7.3 (95% CI 3.2, 16.8) greater in participants whose mothers were diagnosed with diabetes during pregnancy (n = 79; with >90% GDM cases) than in their unexposed counterparts (n = 190) [45]. In addition, in utero exposure to maternal diabetes in conjunction with obesity contributed to 47% of type 2 diabetes cases in adolescent offspring of various race/ethnicities (non-Hispanic White, African American, Hispanic), suggesting that the transgenerational cycle of diabetes begetting diabetes at increasingly younger ages operates in diverse populations and race/ethnic groups. While such findings also reflect the genetic component of type 2 diabetes, the relationship between in utero exposure to maternal diabetes and future risk of type 2 diabetes is robust to adjustment for paternal diabetes and age at onset of diabetes for either parent [46]. Dabelea et al [47] showed further support for a specific intrauterine effect of maternal diabetes on offspring type 2 diabetes risk above and beyond genetics via a discordant sibship analysis wherein the sibling born after maternal diagnosis of diabetes had threefold greater odds of type 2 diabetes than those born before diagnosis.
Pathways linking in utero overnutrition to type 2 diabetes
Although excess adiposity is the leading risk factor for type 2 diabetes in adults [4, 5], whether the relationship between in utero overnutrition and youth-onset type 2 diabetes is a sole consequence of childhood obesity has garnered interest. In the Pima Indian study, acute insulin response to infused glucose was 40% lower in adults whose mothers had diabetes during pregnancy than in those whose mothers developed diabetes after delivery, despite no differences in per cent fat mass [48]. In lower-risk populations, maternal GDM has been associated with precursors of type 2 diabetes in offspring even after accounting for offspring BMI, including higher estimated insulin resistance (HOMA2-IR) among youth in EPOCH [49], and higher fasting glucose among adolescents in the Danish National Birth Cohort [50]. Similarly, in an analysis of 587 mother–offspring dyads in Denmark, Kelstrup et al found that adult offspring exposed to maternal type 1 diabetes or GDM had impaired insulin sensitivity and lower disposition index compared with adult offspring of normoglycaemic women, even after adjustment for BMI [51].
In addition to the effect of overt maternal diabetes on type 2 diabetes in offspring, a recent analysis of 4832 mother–child pairs in the HAPO Follow-Up Study revealed that the entire spectrum of maternal glycaemia (based on maternal fasting glucose levels, as well as plasma glucose levels at 1 h and 2 h post 7 g oral glucose tolerance load) was positively associated with offspring fasting glucose levels and insulin resistance at age 10–14 years, independent of maternal and child BMI and family history of diabetes [9].
Mechanisms underlying the relationship between in utero exposure to maternal diabetes and type 2 diabetes risk in offspring have been gleaned from rodent studies showing a specific detrimental effect of maternal diabetes or hyperglycaemia on offspring pancreatic beta cell development and function [52,53,54]. In addition, small case–control studies of mother–infant pairs with vs without GDM have noted differential expression of genes encoding the insulin receptor [55] and adiponectin [56] in cord blood, independent of maternal BMI, pointing towards epigenetic modifications of specific genes involved in glycaemic regulation as another mechanistic pathway. Further research to identify the exact mechanisms by which in utero exposure to diabetes influences risk of type 2 diabetes in offspring is needed to develop and implement effective prevention.
Maternal obesity
Vohr et al reported that maternal pre-pregnancy weight status and gestational weight gain predicted offspring fat mass at birth [57] and age 1 year [58], even among women without GDM. This positive relationship between maternal weight and offspring adiposity at birth [59] and beyond [49, 60,61,62,63,64] has been confirmed in numerous settings. Moreover, the consequences of pregravid adiposity are detectable across the continuum of maternal BMI and can influence offspring metabolic risk independent of offspring adiposity [64,65,66,67].
The concordance between maternal and offspring obesity can stem from genetics [68], shared environment and lifestyle [69, 70], as well as intrauterine mechanisms. The intrauterine effect of maternal obesity on offspring obesity is difficult to isolate in human studies, but animal experiments support biological plausibility. Diet-induced obesity among pregnant rodents altered offspring adipocyte metabolism to favour hypertrophy via epigenetic modifications [71,72,73,74]. Other pathways include the effect of maternal obesity on placental function [75], glycaemic regulation [76] and stem cell differentiation [77,78,79,80,81]. We include a brief discussion on mechanistic studies using mesenchymal stem cells in the section ‘Mechanistic studies nested within existing cohorts’; other mechanisms are reviewed elsewhere in this issue.
Maternal diet during pregnancy
Macronutrient intake
Studies of maternal macronutrient intake during pregnancy generally indicate that higher energy and carbohydrate intakes and lower protein intakes are associated with higher neonatal adiposity. Specifically, greater carbohydrate intake during late pregnancy was associated with higher neonatal fat mass in a study of 222 Danish mother–child pairs [82], whereas higher protein intake during mid-to-late pregnancy was associated with lower birthweight (Project Viva [83]), neonatal abdominal adiposity (the GUSTO Study [84]) and abdominal fat mass during adolescence (a Danish cohort [85]). In the Healthy Start Study, maternal intake of all energy-providing macronutrients (total fat, saturated fat, unsaturated fat, carbohydrates) except protein was associated with higher neonatal adiposity [86].
Dietary patterns
The field of nutritional epidemiology recently shifted towards evaluating dietary patterns, rather than individual foods or nutrients, to reflect real-life dietary intake [87]. In the Healthy Start Study, poor diet quality during mid-pregnancy, defined as Healthy Eating Index (HEI) score ≤57, corresponded to 0.58% (95% CI: 0.07%, 1.10%) higher fat mass in newborns [88]. A pooled analysis of two cohorts (Project Viva in the USA and the Rhea cohort in Greece) found that adherence to a Mediterranean dietary pattern during mid-pregnancy predicted lower BMI, waist circumference and skinfold thicknesses in offspring across childhood [89]. Yet, a recent study of 721 overweight/obese pregnant women reported no consistent relationship of diet quality indicators, including the HEI, carbohydrate and protein intake and total energy intake, with fetal ultrasound measurements of adiposity at 28–36 weeks’ gestation [90]. While the utility of fetal ultrasounds for assessing neonatal adiposity requires validation, these findings suggest the importance of maternal pre-pregnancy weight status beyond that of diet during pregnancy.
Gestational weight gain
While gestational weight gain (GWG) is not a dietary factor per se, it is a consequence of dietary intake that contributes to the gestational milieu [91,92,93], albeit in conjunction with the influence of pre-pregnancy weight status, and the social, genetic and physiological changes that occur during pregnancy, discussed elsewhere [94]. Both higher GWG on a continuous scale, as well as excess GWG according to current Institute of Medicine guidelines [94], have been consistently related to greater offspring adiposity from birth [95, 96] through adulthood [66, 97, 98]. Many of these studies established this association independent of pre-pregnancy BMI and shared environment/lifestyle factors [64, 97, 98]. GWG is also positively correlated with type 2 diabetes-related metabolic biomarkers in offspring, including insulin resistance and adipocytokine profile [99, 100]. In some cases, the metabolic alterations occurred in the absence of offspring obesity [64, 76].
In addition to the impact of total GWG on offspring adiposity and metabolic profile, the timing of weight gain has repercussions. Greater weight gain assessed continuously during early pregnancy has been linked to higher offspring BMI and fat mass during childhood [101, 102], whereas GWG exceeding current guidelines during the second and third trimesters correlated with greater odds of delivering a large-for-gestational age infant [103]. These discrepancies suggest a need to examine associations of GWG timing with growth trajectories rather than weight status at distinct time points.
Of note, while it is tempting to compare the effects of pre-pregnancy BMI with those of GWG on offspring health, as was done in a recent meta-analysis [104], Gillman [105] rightly pointed out that doing so may not be appropriate given that their relationships with offspring health reflect contributions from different factors at different times across a woman’s lifespan. GWG occurs during pregnancy, exerting influences on offspring health through the intrauterine environment. On the other hand, pre-pregnancy BMI represents the impact of shared genes and environmental factors between mother and child, as well as a direct influence of the in utero environment and postnatal behaviours.
Postnatal overnutrition
Breastfeeding
Exclusive breastfeeding for the first 6 months of life is recommended by the World Health Organization for its protection against infant morbidity [106], benefits to maternal health [106] and potential to reduce childhood obesity [107, 108]. Although some have expressed concern regarding the safety of breastfeeding among diabetic women given the potential for higher levels of insulin and glucose in breast milk [109], studies in diverse populations (e.g. the Pima Indian study [110] and GUTS [111]) reported protective effects of breastfeeding on offspring fat distribution, metabolic traits and type 2 diabetes risk in diabetic and non-diabetic women. Beyond supporting the safety of breastfeeding, Crume et al [112] showed that breastfeeding ≥6 months mitigated the effects of GDM exposure on adiposity at age 6–13 years, suggesting a specific protective effect of breastfeeding among high-risk offspring.
Moving from association to causation to inform clinical care and public health practice
Life course approach
The long latency period between exposure to developmental overnutrition and future obesity/type 2 diabetes necessitates thoughtful study designs and analytical approaches to study disease aetiology, to test mechanisms and to identify opportunities for intervention. Life course epidemiology conceptual models [113] are a valuable tool for these purposes. Conceptual models not only encourage researchers to consider the web of causation among key variables, but also drive the analytical strategy. By accomplishing these tasks, researchers will better understand aetiology and mechanisms, and gain insight into modifiable determinants of type 2 diabetes across the lifespan that both coincide with and occur independently of excess adiposity.
Clever study designs and analytical strategies
Certain study designs can enhance our ability to draw causal conclusions from observational data. For instance, one could minimise the impact of genetics by comparing effect sizes for maternal vs paternal BMI [114], and/or leverage within- vs between-family comparisons [115] and sibship analyses [47, 116]. In addition, new developments in statistical techniques (i.e. inverse proportional weighting of marginal structural models [117, 118]) can enhance causal inference.
Mechanistic studies nested within existing cohorts
In the Healthy Start cohort, in vitro studies of mesenchymal stem cells harvested from cord blood in a sample of study participants provided evidence that alterations in β-catenin pathways, expression of genes involved in myocyte growth, amino acid synthesis and oxidative stress link maternal obesity to newborn adiposity and weight gain during infancy [77,78,79,80,81]. Such findings support biological plausibility of observational findings of the positive correlation between maternal and child obesity in this and other cohorts.
Randomised clinical trials
Most randomised clinical trials comprising interventions focused on obesogenic conditions during pregnancy have not been effective in mitigating/reducing GDM or excess GWG [119], or preventing macrosomia [119, 120]. The limited success of these trials point towards a need for interventions prior to conception or earlier in pregnancy. Pre-conception interventions may be difficult to implement among first-time mothers given that half of pregnancies are unplanned [121], and early pregnancy interventions may be similarly challenging given that most women seek prenatal care midway through the first trimester. A potential strategy is to focus on women with a history of obesity, GDM and/or excessive GWG for surveillance and interventions prior to the next pregnancy [122]. In light of several recent studies indicating the importance of the first trimester to both maternal [123] and offspring health [101, 102], interventions on maternal behaviours to moderate weight gain during the first trimester seem promising.
There is also room for improvement in the endpoints targeted by pregnancy trials. For example, directly measured neonatal fat mass rather than birthweight is likely to be more relevant to the development of obesity and type 2 diabetes. Furthermore, longer follow-up of health outcomes beyond birth is warranted to better understand the efficacy of pregnancy trials on chronic disease risk prevention.
Finally, following findings that adequate breastfeeding limits the detrimental effects of GDM exposure [112], promoting breastfeeding among diabetic women may help to reduce obesity-related conditions in high-risk offspring, hopefully breaking the intergenerational cycle of disease.
Summary and conclusions
Youth-onset type 2 diabetes is on the rise, and trends in childhood obesity only partially explain the recent appearance of a condition that was previously confined to adults. Higher maternal BMI entering pregnancy (not simply maternal obesity), maternal hyperglycaemia (even in the absence of overt diabetes), greater GWG (not only excessive GWG according to current guidelines), and greater energy intake during pregnancy are important early-life correlates of excess adiposity and youth-onset type 2 diabetes. Importantly, maternal hyperglycaemia and GDM are associated with precursors of type 2 diabetes (e.g. insulin resistance, reduced disposition index) in offspring, starting as early as late childhood, even after accounting for current body size and/or adiposity, suggesting a specific effect of maternal hyperglycaemia on pancreatic beta cell development and function. Given the overlapping nature of these aspects of developmental overnutrition, and the shared aetiology of obesity and type 2 diabetes, future studies are warranted to disentangle pathways linking specific aspects of developmental overnutrition to obesity and type 2 diabetes risk. Accomplishment of these tasks will inform timing and targets for early interventions with potential to make measurable impacts on population health.
Abbreviations
- EPOCH:
-
Exploring Perinatal Outcomes in Children
- GDM:
-
Gestational diabetes mellitus
- GUTS:
-
Growing Up Today Study
- GWG:
-
Gestational weight gain
- HAPO:
-
Hyperglycemia and Adverse Pregnancy Outcomes
- HEI:
-
Healthy Eating Index
References
Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC (2018) Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics 141(3):e20173459. https://doi.org/10.1542/peds.2017-3459
Mayer-Davis EJ, Lawrence JM, Dabelea D et al (2017) Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 376(15):1419–1429. https://doi.org/10.1056/NEJMoa1610187
D’Adamo E, Caprio S (2011) Type 2 diabetes in youth: epidemiology and pathophysiology. Diabetes Care 34(Suppl 2):S161–S165. https://doi.org/10.2337/dc11-s212
Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC (1994) Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 17(9):961–969. https://doi.org/10.2337/diacare.17.9.961
Carey VJ, Walters EE, Colditz GA et al (1997) Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am J Epidemiol 145(7):614–619. https://doi.org/10.1093/oxfordjournals.aje.a009158
Pedersen J (1971) Diabetes mellitus and pregnancy: present status of the hyperglycaemia–hyperinsulinism theory and the weight of the newborn baby. Postgrad Med J 1971(Suppl):66–67
Dabelea D, Harrod CS (2013) Role of developmental overnutrition in pediatric obesity and type 2 diabetes. Nutr Rev 71(Suppl 1):S62–S67. https://doi.org/10.1111/nure.12061
Dabelea D, Crume T (2011) Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes 60(7):1849–1855. https://doi.org/10.2337/db11-0400
Scholtens DM, Kuang A, Lowe LP et al (2019) Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal glycemia and childhood glucose metabolism. Diabetes Care 42(3):381–392. https://doi.org/10.2337/dc18-2021
Dabelea D, Hanson RL, Bennett PH, Roumain J, Knowler WC, Pettitt DJ (1998) Increasing prevalence of Type II diabetes in American Indian children. Diabetologia 41(8):904–910. https://doi.org/10.1007/s001250051006
Berk MA, Mimouni F, Miodovnik M, Hertzberg V, Valuck J (1989) Macrosomia in infants of insulin-dependent diabetic mothers. Pediatrics 83(6):1029–1034
Kc K, Shakya S, Zhang H (2015) Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab 66(Suppl 2):14–20. https://doi.org/10.1159/000371628
Ladfors L, Shaat N, Wiberg N, Katasarou A, Berntorp K, Kristensen K (2017) Fetal overgrowth in women with type 1 and type 2 diabetes mellitus. PLoS One 12(11):e0187917. https://doi.org/10.1371/journal.pone.0187917
Lampl M, Jeanty P (2004) Exposure to maternal diabetes is associated with altered fetal growth patterns: a hypothesis regarding metabolic allocation to growth under hyperglycemic-hypoxemic conditions. Am J Hum Biol 16(3):237–263. https://doi.org/10.1002/ajhb.20015
Catalano PM, Thomas A, Huston-Presley L, Amini SB (2003) Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol 189(6):1698–1704. https://doi.org/10.1016/S0002-9378(03)00828-7
Crume TL, Shapiro AL, Brinton JT et al (2015) Maternal fuels and metabolic measures during pregnancy and neonatal body composition: The Healthy Start Study. J Clin Endocrinol Metab 100(4):1672–1680. https://doi.org/10.1210/jc.2014-2949
Yogev Y, Langer O, Xenakis EM, Rosenn B (2005) The association between glucose challenge test, obesity and pregnancy outcome in 6390 non-diabetic women. J Matern Fetal Neonatal Med 17(1):29–34. https://doi.org/10.1080/14767050400028766
Metzger BE, Lowe LP, Dyer AR et al (2008) Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 358(19):1991–2002. https://doi.org/10.1056/NEJMoa0707943
Gomes D, von Kries R, Delius M et al (2018) Late-pregnancy dysglycemia in obese pregnancies after negative testing for gestational diabetes and risk of future childhood overweight: an interim analysis from a longitudinal mother–child cohort study. PLoS Med 15(10):e1002681. https://doi.org/10.1371/journal.pmed.1002681
Jones AI, Coleman EL, Husni NR et al (2017) Type 1 diabetes alters lipid handling and metabolism in human fibroblasts and peripheral blood mononuclear cells. PLoS One 12(12):e0188474. https://doi.org/10.1371/journal.pone.0188474
Ji J, Petropavlovskaia M, Khatchadourian A et al (2019) Type 2 diabetes is associated with suppression of autophagy and lipid accumulation in β-cells. J Cell Mol Med 23(4):2890–2900. https://doi.org/10.1111/jcmm.14172
Ryckman K, Spracklen C, Smith C, Robinson J, Saftlas A (2015) Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis. BJOG 122(5):643–651. https://doi.org/10.1111/1471-0528.13261
Tontonoz P, Hu E, Spiegelman BM (1994) Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 79(7):1147–1156. https://doi.org/10.1016/0092-8674(94)90006-X
Schaefer-Graf UM, Meitzner K, Ortega-Senovilla H et al (2011) Differences in the implications of maternal lipids on fetal metabolism and growth between gestational diabetes mellitus and control pregnancies. Diabet Med 28(9):1053–1059. https://doi.org/10.1111/j.1464-5491.2011.03346.x
Schaefer-Graf UM, Graf K, Kulbacka I et al (2008) Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 31(9):1858–1863. https://doi.org/10.2337/dc08-0039
Simeonova-Krstevska S, Krstevska B, Velkoska-Nakova V et al (2014) Effect of lipid parameters on foetal growth in gestational diabetes mellitus pregnancies. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 35(2):131–136
Misra VK, Trudeau S, Perni U (2011) Maternal serum lipids during pregnancy and infant birth weight: the influence of prepregnancy BMI. Obesity (Silver Spring, Md) 19(7):1476–1481. https://doi.org/10.1038/oby.2011.43
Kulkarni SR, Kumaran K, Rao SR et al (2013) Maternal lipids are as important as glucose for fetal growth: findings from the Pune Maternal Nutrition Study. Diabetes Care 36(9):2706–2713. https://doi.org/10.2337/dc12-2445
Harmon KA, Gerard L, Jensen DR et al (2011) Continuous glucose profiles in obese and normal-weight pregnant women on a controlled diet: metabolic determinants of fetal growth. Diabetes Care 34(10):2198–2204. https://doi.org/10.2337/dc11-0723
Clausen T, Burski TK, Oyen N, Godang K, Bollerslev J, Henriksen T (2005) Maternal anthropometric and metabolic factors in the first half of pregnancy and risk of neonatal macrosomia in term pregnancies. A prospective study. Eur J Endocrinol 153(6):887–894. https://doi.org/10.1530/eje.1.02034
Newgard CB (2012) Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 15(5):606–614. https://doi.org/10.1016/j.cmet.2012.01.024
Scholtens DM, Muehlbauer MJ, Daya NR et al (2014) Metabolomics reveals broad-scale metabolic perturbations in hyperglycemic mothers during pregnancy. Diabetes Care 37(1):158–166. https://doi.org/10.2337/dc13-0989
Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA (2003) Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 111(3):e221–e226. https://doi.org/10.1542/peds.111.3.e221
Regnault N, Gillman MW, Rifas-Shiman SL, Eggleston E, Oken E (2013) Sex-specific associations of gestational glucose tolerance with childhood body composition. Diabetes Care 36(10):3045–3053. https://doi.org/10.2337/dc13-0333
Crume TL, Ogden L, West NA et al (2011) Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) study. Diabetologia 54(1):87–92. https://doi.org/10.1007/s00125-010-1925-3
Pitchika A, Vehik K, Hummel S et al (2018) Associations of maternal diabetes during pregnancy with overweight in offspring: results from the Prospective TEDDY Study. Obesity (Silver Spring, Md) 26(9):1457–1466. https://doi.org/10.1002/oby.22264
Kawasaki M, Arata N, Miyazaki C et al (2018) Obesity and abnormal glucose tolerance in offspring of diabetic mothers: A systematic review and meta-analysis. PLoS One 13(1):e0190676. https://doi.org/10.1371/journal.pone.0190676
Kim SY, England JL, Sharma JA, Njoroge T (2011) Gestational diabetes mellitus and risk of childhood overweight and obesity in offspring: a systematic review. Exp Diabetes Res 2011:541308–541309. https://doi.org/10.1155/2011/541308
Philipps LH, Santhakumaran S, Gale C et al (2011) The diabetic pregnancy and offspring BMI in childhood: a systematic review and meta-analysis. Diabetologia 54(8):1957–1966. https://doi.org/10.1007/s00125-011-2180-y
Hernán MA, Hernández-Díaz S, Robins JM (2004) A structural approach to selection bias. Epidemiology 15(5):615–625. https://doi.org/10.1097/01.ede.0000135174.63482.43
Bennett PH, Rushforth NB, Miller M, LeCompte PM (1976) Epidemiologic studies of diabetes in the Pima Indians. Recent Prog Horm Res 32:333–376
Pettitt DJ, Baird HR, Aleck KA, Bennett PH, Knowler WC (1983) Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med 308(5):242–245. https://doi.org/10.1056/nejm198302033080502
Petitt DJ, Bennett PH, Knowler WC, Baird HR, Aleck KA (1985) Gestational diabetes mellitus and impaired glucose tolerance during pregnancy. Long-term effects on obesity and glucose tolerance in the offspring. Diabetes 34(Suppl 2):119–122. https://doi.org/10.2337/diab.34.2.S119
Dabelea D, Knowler WC, Pettitt DJ (2000) Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med 9(1):83–88. https://doi.org/10.1002/(sici)1520-6661(200001/02)9:1<83::aid-mfm17>3.0.co;2-o
Dabelea D, Mayer-Davis EJ, Lamichhane AP et al (2008) Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care 31(7):1422–1426. https://doi.org/10.2337/dc07-2417
Pettitt D (1996) Diabetes in subsequent generations. In: Doornhorst A, Hadden DR (eds) Diabetes and pregnancy: an international approach to diagnosis and management. John Wiley & Sons, Chichester, pp 367–376
Dabelea D, Hanson RL, Lindsay RS et al (2000) Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 49(12):2208–2211. https://doi.org/10.2337/diabetes.49.12.2208
Gautier JF, Wilson C, Weyer C et al (2001) Low acute insulin secretory responses in adult offspring of people with early onset type 2 diabetes. Diabetes 50(8):1828–1833. https://doi.org/10.2337/diabetes.50.8.1828
Sauder KA, Hockett CW, Ringham BM, Glueck DH, Dabelea D (2017) Fetal overnutrition and offspring insulin resistance and beta-cell function: the Exploring Perinatal Outcomes among Children (EPOCH) study. Diabet Med 34(10):1392–1399. https://doi.org/10.1111/dme.13417
Grunnet LG, Hansen S, Hjort L et al (2017) Adiposity, dysmetabolic traits, and earlier onset of female puberty in adolescent offspring of women with gestational diabetes mellitus: a clinical study within the Danish National Birth Cohort. Diabetes Care 40(12):1746–1755. https://doi.org/10.2337/dc17-0514
Kelstrup L, Damm P, Mathiesen ER et al (2013) Insulin resistance and impaired pancreatic beta-cell function in adult offspring of women with diabetes in pregnancy. J Clin Endocrinol Metab 98(9):3793–3801. https://doi.org/10.1210/jc.2013-1536
Han J, Xu J, Long YS, Epstein PN, Liu YQ (2007) Rat maternal diabetes impairs pancreatic beta-cell function in the offspring. Am J Physiol Endocrinol Metab 293(1):E228–E236. https://doi.org/10.1152/ajpendo.00479.2006
Aerts L, Van Assche FA (2006) Animal evidence for the transgenerational development of diabetes mellitus. Int J Biochem Cell Biol 38(5–6):894–903. https://doi.org/10.1016/j.biocel.2005.07.006
Aerts L, Sodoyez-Goffaux F, Sodoyez JC, Malaisse WJ, Van Assche FA (1988) The diabetic intrauterine milieu has a long-lasting effect on insulin secretion by B cells and on insulin uptake by target tissues. Am J Obstet Gynecol 159(5):1287–1292. https://doi.org/10.5555/uri:pii:0002937888904656
Ott R, Melchior K, Stupin JH et al (2019) Reduced insulin receptor expression and altered DNA methylation in fat tissues and blood of women with GDM and offspring. J Clin Endocrinol Metab 104(1):137–149. https://doi.org/10.1210/jc.2018-01659
Ott R, Stupin JH, Melchior K et al (2018) Alterations of adiponectin gene expression and DNA methylation in adipose tissues and blood cells are associated with gestational diabetes and neonatal outcome. Clin Epigenetics 10(1):131. https://doi.org/10.1186/s13148-018-0567-z
Vohr BR, McGarvey ST, Coll CG (1995) Effects of maternal gestational diabetes and adiposity on neonatal adiposity and blood pressure. Diabetes Care 18(4):467–475. https://doi.org/10.2337/diacare.18.4.467
Vohr BR, McGarvey ST (1997) Growth patterns of large-for-gestational-age and appropriate-for-gestational-age infants of gestational diabetic mothers and control mothers at age 1 year. Diabetes Care 20(7):1066–1072. https://doi.org/10.2337/diacare.20.7.1066
Starling AP, Brinton JT, Glueck DH et al (2015) Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start Study. Am J Clin Nutr 101(2):302–309. https://doi.org/10.3945/ajcn.114.094946
Sauder KA, Kaar JL, Starling AP, Ringham BM, Glueck DH, Dabelea D (2017) Predictors of infant body composition at 5 months of age: the Healthy Start Study. J Pediatr 183:94–99.e91. https://doi.org/10.1016/j.jpeds.2017.01.014
Shapiro AL, Schmiege SJ, Brinton JT et al (2015) Testing the fuel-mediated hypothesis: maternal insulin resistance and glucose mediate the association between maternal and neonatal adiposity, the Healthy Start study. Diabetologia 58(5):937–941. https://doi.org/10.1007/s00125-015-3505-z
Sharp GC, Lawlor DA, Richmond RC et al (2015) Maternal pre-pregnancy BMI and gestational weight gain, offspring DNA methylation and later offspring adiposity: findings from the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 44(4):1288–1304. https://doi.org/10.1093/ije/dyv042
Santos S, Monnereau C, Felix JF, Duijts L, Gaillard R, Jaddoe VWV (2018) Maternal body mass index, gestational weight gain, and childhood abdominal, pericardial, and liver fat assessed by magnetic resonance imaging. Int J Obes 43(3):581–593. https://doi.org/10.1038/s41366-018-0186-y
Perng W, Gillman MW, Mantzoros CS, Oken E (2014) A prospective study of maternal prenatal weight and offspring cardiometabolic health in midchildhood. Ann Epidemiol 24(11):793–800.e1. https://doi.org/10.1016/j.annepidem.2014.08.002
Lemas DJ, Brinton JT, Shapiro AL, Glueck DH, Friedman JE, Dabelea D (2015) Associations of maternal weight status prior and during pregnancy with neonatal cardiometabolic markers at birth: the Healthy Start study. Int J Obes 39(10):1437–1442. https://doi.org/10.1038/ijo.2015.109
Hochner H, Friedlander Y, Calderon-Margalit R et al (2012) Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow-up Study. Circulation 125(11):1381–1389. https://doi.org/10.1161/circulationaha.111.070060
Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X (2013) Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One 8(4):e61627–e61627. https://doi.org/10.1371/journal.pone.0061627
Maes HH, Neale MC, Eaves LJ (1997) Genetic and environmental factors in relative body weight and human adiposity. Behav Genet 27(4):325–351. https://doi.org/10.1023/A:1025635913927
Scaglioni S, Arrizza C, Vecchi F, Tedeschi S (2011) Determinants of children’s eating behavior. Am J Clin Nutr 94(6 Suppl):2006s–2011s. https://doi.org/10.3945/ajcn.110.001685
Robinson SM (2005) Godfrey KM (2008) feeding practices in pregnancy and infancy: relationship with the development of overweight and obesity in childhood. Int J Obes 32(Suppl 6):S4–S10. https://doi.org/10.1038/ijo.2008.201
Begum G, Davies A, Stevens A et al (2013) Maternal undernutrition programs tissue-specific epigenetic changes in the glucocorticoid receptor in adult offspring. Endocrinology. 154(12):4560–4569. https://doi.org/10.1210/en.2013-1693
Samuelsson AM, Matthews PA, Argenton M et al (2008) Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51(2):383–392. https://doi.org/10.1161/HYPERTENSIONAHA.107.101477
Bringhenti I, Moraes-Teixeira JA, Cunha MR, Ornellas F, Mandarim-de-Lacerda CA, Aguila MB (2013) Maternal obesity during the preconception and early life periods alters pancreatic development in early and adult life in male mouse offspring. PLoS One 8(1):e55711. https://doi.org/10.1371/journal.pone.0055711
Howie GJ, Sloboda DM, Kamal T, Vickers MH (2009) Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol 587(Pt 4):905–915. https://doi.org/10.1113/jphysiol.2008.163477
Howell KR, Powell TL (2017) Effects of maternal obesity on placental function and fetal development. Reproduction 153(3):R97–R108. https://doi.org/10.1530/REP-16-0495
Mingrone G, Manco M, Mora MEV et al (2008) Influence of maternal obesity on insulin sensitivity and secretion in offspring. Diabetes Care 31(9):1872–1876. https://doi.org/10.2337/dc08-0432
Boyle KE, Patinkin ZW, Shapiro ALB et al (2017) Maternal obesity alters fatty acid oxidation, AMPK activity, and associated DNA methylation in mesenchymal stem cells from human infants. Mol Metab 6(11):1503–1516. https://doi.org/10.1016/j.molmet.2017.08.012
Boyle KE, Patinkin ZW, Shapiro AL, Baker PR 2nd, Dabelea D, Friedman JE (2016) Mesenchymal stem cells from infants born to obese mothers exhibit greater potential for adipogenesis: The Healthy Start BabyBUMP project. Diabetes 65(3):647–659. https://doi.org/10.2337/db15-0849
Baker PR 2nd, Patinkin ZW, Shapiro ALB et al (2017) Altered gene expression and metabolism in fetal umbilical cord mesenchymal stem cells correspond with differences in 5-month-old infant adiposity gain. Sci Rep 7(1):18095. https://doi.org/10.1038/s41598-017-17588-4
Baker PR, 2nd, Patinkin Z, Shapiro AL, et al. (2017) Maternal obesity and increased neonatal adiposity correspond with altered infant mesenchymal stem cell metabolism. JCI Insight 2(21).pii: 94200. https://doi.org/10.1172/jci.insight.94200
Shapiro AL, Boyle KE, Dabelea D et al (2016) Nicotinamide promotes adipogenesis in umbilical cord-derived mesenchymal stem cells and is associated with neonatal adiposity: The Healthy Start BabyBUMP Project. PLoS One 11(7):e0159575. https://doi.org/10.1371/journal.pone.0159575
Renault KM, Carlsen EM, Norgaard K et al (2015) Intake of carbohydrates during pregnancy in obese women is associated with fat mass in the newborn offspring. Am J Clin Nutr 102(6):1475–1481. https://doi.org/10.3945/ajcn.115.110551
Switkowski KM, Jacques PF, Must A, Kleinman KP, Gillman MW, Oken E (2016) Maternal protein intake during pregnancy and linear growth in the offspring. Am J Clin Nutr 104(4):1128–1136. https://doi.org/10.3945/ajcn.115.128421
Chen LW, Tint MT, Fortier MV et al (2016) Maternal macronutrient intake during pregnancy is associated with neonatal abdominal adiposity: The Growing Up in Singapore Towards healthy Outcomes (GUSTO) Study. J Nutr 146(8):1571–1579. https://doi.org/10.3945/jn.116.230730
Maslova E, Hansen S, Grunnet LG et al (2017) Maternal protein intake in pregnancy and offspring metabolic health at age 9–16 y: results from a Danish cohort of gestational diabetes mellitus pregnancies and controls. Am J Clin Nutr 106(2):623–636. https://doi.org/10.3945/ajcn.115.128637
Crume TL, Brinton JT, Shapiro A et al (2016) Maternal dietary intake during pregnancy and offspring body composition: The Healthy Start Study. Am J Obstet Gynecol 215(5):609.e601–609.e608. https://doi.org/10.1016/j.ajog.2016.06.035
Hu FB (2002) Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 13(1):3–9. https://doi.org/10.1097/00041433-200202000-00002
Shapiro AL, Kaar JL, Crume TL et al (2016) Maternal diet quality in pregnancy and neonatal adiposity: the Healthy Start Study. Int J Obes (2005) 40(7):1056–1062. https://doi.org/10.1038/ijo.2016.79
Chatzi L, Rifas-Shiman SL, Georgiou V et al (2017) Adherence to the Mediterranean diet during pregnancy and offspring adiposity and cardiometabolic traits in childhood. Pediatric obesity 12(Suppl 1):47–56. https://doi.org/10.1111/ijpo.12191
O’Brien CM, Louise J, Deussen A, Dodd JM (2018) In Overweight or obese pregnant women, maternal dietary factors are not associated with fetal growth and adiposity. Nutrients 10(7):870. https://doi.org/10.3390/nu10070870
Tobias D, Bao W (2014) Diet during pregnancy and gestational weight gain. Curr Nutri Rep 3(3):289–297. https://doi.org/10.1007/s13668-014-0092-4
Hrolfsdottir L, Schalkwijk CG, Birgisdottir BE et al (2016) Maternal diet, gestational weight gain, and inflammatory markers during pregnancy. Obesity (Silver Spring, Md) 24(10):2133–2139. https://doi.org/10.1002/oby.21617
Shin D, Bianchi L, Chung H, Weatherspoon L, Song WO (2014) Is gestational weight gain associated with diet quality during pregnancy? Matern Child Health J 18(6):1433–1443. https://doi.org/10.1007/s10995-013-1383-x
Institute of Medicine National Research Council Committee to Reexamine IOM Pregnancy Weight Guidelines (2009) In: Rasmussen KM, Yaktine AL (eds) Weight gain during pregnancy: reexamining the guidelines. Washington, National Academies Press (US)
Deierlein AL, Siega-Riz AM, Adair LS, Herring AH (2011) Effects of pre-pregnancy body mass index and gestational weight gain on infant anthropometric outcomes. J Pediatr 158(2):221–226. https://doi.org/10.1016/j.jpeds.2010.08.008
Blackwell SC, Landon MB, Mele L et al (2016) Relationship between excessive gestational weight gain and neonatal adiposity in women with mild gestational diabetes mellitus. Obstet Gynecol 128(6):1325–1332. https://doi.org/10.1097/AOG.0000000000001773
Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW (2007) Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol 196(4):322.e321–322.e328. https://doi.org/10.1016/j.ajog.2006.11.027
Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW (2008) Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol 112(5):999–1006. https://doi.org/10.1097/AOG.0b013e31818a5d50
Dello Russo M, Ahrens W, De Vriendt T et al (2013) Gestational weight gain and adiposity, fat distribution, metabolic profile, and blood pressure in offspring: the IDEFICS project. Int J Obes (2005) 37(7):914–919. https://doi.org/10.1038/ijo.2013.35
Fraser A, Tilling K, Macdonald-Wallis C et al (2011) Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: the Avon Longitudinal Study of Parents and Children (ALSPAC). Am J Clin Nutr 93(6):1285–1292. https://doi.org/10.3945/ajcn.110.008326
Margerison-Zilko CE, Shrimali BP, Eskenazi B, Lahiff M, Lindquist AR, Abrams BF (2012) Trimester of maternal gestational weight gain and offspring body weight at birth and age five. Matern Child Health J 16(6):1215–1223. https://doi.org/10.1007/s10995-011-0846-1
Hivert MF, Rifas-Shiman SL, Gillman MW, Oken E (2016) Greater early and mid-pregnancy gestational weight gains are associated with excess adiposity in mid-childhood. Obesity (Silver Spring, Md) 24(7):1546–1553. https://doi.org/10.1002/oby.21511
Sridhar SB, Xu F, Hedderson MM (2016) Trimester-Specific gestational weight gain and infant size for gestational age. PLoS One 11(7):e0159500–e0159500. https://doi.org/10.1371/journal.pone.0159500
Voerman E, Santos S, Patro Golab B et al (2019) Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: an individual participant data meta-analysis. PLoS Med 16(2):e1002744. https://doi.org/10.1371/journal.pmed.1002744
Gillman MW (2012) Gestational weight gain: now and the future. Circulation 125(11):1339–1340. https://doi.org/10.1161/circulationaha.112.091751
Kramer MS, Kakuma R (2012) Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev, Issue 8, Art. no.: CD003517. https://doi.org/10.1002/14651858.CD003517.pub2
World Health Organization (2014) Exclusive breastfeeding to reduce the risk of childhood overweight and obesity. Available from www.who.int/elena/titles/bbc/breastfeeding_childhood_obesity/en/. Accessed 7 April 2019
Oken E, Fields DA, Lovelady CA, Redman LM (2017) TOS Scientific Position Statement: Breastfeeding and Obesity. Obesity (Silver Spring, Md) 25(11):1864–1866. https://doi.org/10.1002/oby.22024
Jovanovic-Peterson L, Fuhrmann K, Hedden K, Walker L, Peterson CM (1989) Maternal milk and plasma glucose and insulin levels: studies in normal and diabetic subjects. J Am Coll Nutr 8(2):125–131. https://doi.org/10.1080/07315724.1989.10720287
Pettitt DJ, Knowler WC (1998) Long-term effects of the intrauterine environment, birth weight, and breast-feeding in Pima Indians. Diabetes Care 21(Suppl 2):B138–B141
Mayer-Davis EJ, Rifas-Shiman SL, Zhou L, Hu FB, Colditz GA, Gillman MW (2006) Breast-feeding and risk for childhood obesity: does maternal diabetes or obesity status matter? Diabetes Care 29(10):2231–2237. https://doi.org/10.2337/dc06-0974
Crume TL, Ogden L, Maligie M et al (2011) Long-term impact of neonatal breastfeeding on childhood adiposity and fat distribution among children exposed to diabetes in utero. Diabetes Care 34(3):641–645. https://doi.org/10.2337/dc10-1716
Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C (2003) Life course epidemiology. J Epidemiol Community Health 57(10):778–783. https://doi.org/10.1136/jech.57.10.778
Gaillard R, Steegers EA, Duijts L et al (2014) Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the Generation R Study. Hypertension 63(4):683–691. https://doi.org/10.1161/hypertensionaha.113.02671
Branum AM, Parker JD, Keim SA, Schempf AH (2011) Prepregnancy body mass index and gestational weight gain in relation to child body mass index among siblings. Am J Epidemiol 174(10):1159–1165. https://doi.org/10.1093/aje/kwr250
Villamor E, Cnattingius S (2006) Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet 368(9542):1164–1170. https://doi.org/10.1016/S0140-6736(06)69473-7
Young JG, Hernan MA, Picciotto S, Robins JM (2010) Relation between three classes of structural models for the effect of a time-varying exposure on survival. Lifetime Data Anal 16(1):71–84. https://doi.org/10.1007/s10985-009-9135-3
Snowden JM, Rose S, Mortimer KM (2011) Implementation of G-computation on a simulated data set: demonstration of a causal inference technique. Am J Epidemiol 173(7):731–738. https://doi.org/10.1093/aje/kwq472
Dodd JM, Grivell RM, Crowther CA, Robinson JS (2010) Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials. BJOG 117(11):1316–1326. https://doi.org/10.1111/j.1471-0528.2010.02540.x
Dodd JM, Deussen AR, Mohamad I et al (2016) The effect of antenatal lifestyle advice for women who are overweight or obese on secondary measures of neonatal body composition: the LIMIT randomised trial. BJOG 123(2):244–253. https://doi.org/10.1111/1471-0528.13796
Finer LB, Zolna MR (2016) Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med 374(9):843–852. https://doi.org/10.1056/NEJMsa1506575
Ratner RE, Christophi CA, Metzger BE et al (2008) Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 93(12):4774–4779. https://doi.org/10.1210/jc.2008-0772
Hedderson MM, Gunderson EP, Ferrara A (2010) Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol 115(3):597–604. https://doi.org/10.1097/AOG.0b013e3181cfce4f
Author information
Authors and Affiliations
Contributions
WP and DD conceptualised the review. WP wrote the initial draft of the manuscript, incorporated changes suggested by co-authors, and formatted the paper for publication. DD and EO provided critical intellectual feedback. All authors approved the version of the paper to be published.
Corresponding author
Ethics declarations
The authors declare that there is no duality of interest associated with this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Figure slide
(PPTX 201 kb)
Rights and permissions
About this article
Cite this article
Perng, W., Oken, E. & Dabelea, D. Developmental overnutrition and obesity and type 2 diabetes in offspring. Diabetologia 62, 1779–1788 (2019). https://doi.org/10.1007/s00125-019-4914-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-019-4914-1