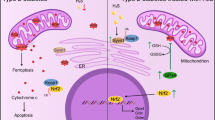
Abstract
Hydrogen sulfide (H2S) is involved in diverse physiological functions, such as anti-hypertension, anti-proliferation, regulating ATP synthesis, and reactive oxygen species production. Sirtuin 3 (SIRT3) is a NAD+-dependent deacetylase that regulates mitochondrial energy metabolism. The role of H2S in energy metabolism in diabetic cardiomyopathy (DCM) may be related to regulate SIRT3 expression; however, this role remains to be elucidated. We hypothesized that exogenous H2S could switch cardiac energy metabolic substrate preference by lysine acetylation through promoting the expression of SIRT3 in cardiac tissue of db/db mice. Db/db mice, neonatal rat cardiomyocytes, and H9c2 cell line with the treatment of high glucose, oleate, and palmitate were used as animal and cellular models of type 2 diabetes. Using LC-MS/MS, we identified 76 proteins that increased acetylation, including 8 enzymes related to fatty acid β-oxidation and 7 enzymes of the tricarboxylic acid (TCA) cycle in the db/db mice hearts compared to those with the treatment of NaHS. Exogenous H2S restored the expression of NAMPT and the ratio of NAD+/NADH enhanced the expression and activity of SIRT3. As a result of activation of SIRT3, the acetylation level and activity of fatty acid β-oxidation enzyme LCAD and the acetylation of glucose oxidation enzymes PDH, IDH2, and CS were reduced which resulted in activation of PDH, IDH2, and CS. Our finding suggested that H2S induced a switch in cardiac energy substrate utilization from fatty acid β-oxidation to glucose oxidation in DCM through regulating SIRT3 pathway.
Key messages
-
H2S regulated the acetylation level and activities of enzymes in fatty acid oxidation and glucose oxidation in cardiac tissues of db/db mice.
-
Exogenous H2S decreased mitochondrial acetylation level through upregulating the expression and activity of SIRT3 in vivo and in vitro.
-
H2S induced a switch in cardiac energy substrate utilization from fatty acid oxidation to glucose.









Similar content being viewed by others
Abbreviations
- C-7Az:
-
7-Azido-4-methylcoumarin
- CPT1:
-
Carnitine palmitoyltransferase 1
- CS:
-
Citrate synthase
- CSE:
-
Cystathionine gamma-lyase
- DCM:
-
Diabetic cardiomyopathy
- ESM:
-
Electronic supplementary material
- FAO:
-
Fatty acid β-oxidation
- FFA:
-
Free fatty acid
- GO:
-
Gene ontology
- H2S:
-
Hydrogen sulfide
- IDH:
-
Isocitrate dehydrogenase
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- LCAD:
-
Long-chain acyl-CoA dehydrogenase
- Lys:
-
Lysine
- NAD:
-
Nicotinamide adenine dinucleotides
- NAM:
-
Nicotinamide
- NAMPT:
-
Nicotinamide phosphoribosyltransferase
- NMN:
-
Nicotinamide mononucleotide
- PDH:
-
Pyruvate dehydrogenase
- PPG:
-
DL-propargylglycine
- RCR:
-
Respiratory control rate
- SIRT:
-
Silent mating type information regulation 2 homolog
- SIRT 3:
-
Sirtuin 3
- TCA:
-
Tricarboxylic acid cycle
- T2DM:
-
Type 2 diabetes mellitus
References
Chen L, Magliano DJ, Zimmet PZ (2011) The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol 8(4):228–236
Roger VL (2013) Epidemiology of heart failure. Circ Res 113(6):646–659
Poirier P, Bogaty P, Garneau C, Marois L, Dumesnil JG (2001) Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care 24(1):5–10
Kiencke S, Handschin R, von Dahlen R, Muser J, Brunner-Larocca HP, Schumann J, Felix B, Berneis K, Rickenbacher P (2010) Pre-clinical diabetic cardiomyopathy: prevalence, screening, and outcome. Eur J Heart Fail 12(9):951–957
Fukushima A, Lopaschuk GD (2016) Acetylation control of cardiac fatty acid beta-oxidation and energy metabolism in obesity, diabetes, and heart failure. Biochim Biophys Acta
Lopaschuk GD, Folmes CD, Stanley WC (2007) Cardiac energy metabolism in obesity. Circ Res 101(4):335–347
Ussher JR (2014) The role of cardiac lipotoxicity in the pathogenesis of diabetic cardiomyopathy. Expert Rev Cardiovasc Ther 12(3):345–358
Fukushima A, Lopaschuk GD (2016) Cardiac fatty acid oxidation in heart failure associated with obesity and diabetes. Biochim Biophys Acta 1860(10):1525–1534
Stanley WC, Recchia FA, Lopaschuk GD (2005) Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85(3):1093–1129
Lantier L, Williams AS, Williams IM, Yang KK, Bracy DP, Goelzer M, James FD, Gius D, Wasserman DH (2015) SIRT3 is crucial for maintaining skeletal muscle insulin action and protects against severe insulin resistance in high-fat-fed mice. Diabetes 64(9):3081–3092
Verdin E, Hirschey MD, Finley LW, Haigis MC (2010) Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci 35(12):669–675
Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T (2008) A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A 105(38):14447–14452
Vassilopoulos A, Pennington JD, Andresson T, Rees DM, Bosley AD, Fearnley IM, Ham A, Flynn CR, Hill S, Rose KL, Kim HS, Deng CX, Walker JE, Gius D (2014) SIRT3 deacetylates ATP synthase F1 complex proteins in response to nutrient- and exercise-induced stress. Antioxid Redox Signal 21(4):551–564
Fu M, Zhang W, Wu L, Yang G, Li H, Wang R (2012) Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc Natl Acad Sci U S A 109(8):2943–2948
Ostrakhovitch EA, Akakura S, Sanokawa-Akakura R, Goodwin S, Tabibzadeh S (2015) Dedifferentiation of cancer cells following recovery from a potentially lethal damage is mediated by H2S-Nampt. Exp Cell Res 330(1):135–150
Zhong G, Chen F, Cheng Y, Tang C, Du J (2003) The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J Hypertens 21(10):1879–1885
Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R (2008) H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322(5901):587–590
Kang K, Zhao M, Jiang H, Tan G, Pan S, Sun X (2009) Role of hydrogen sulfide in hepatic ischemia-reperfusion-induced injury in rats. Liver Transpl 15(10):1306–1314
Chen B, Li W, Lv C, Zhao M, Jin H, Du J, Zhang L, Tang X (2013) Fluorescent probe for highly selective and sensitive detection of hydrogen sulfide in living cells and cardiac tissues. Analyst 138(3):946–951
Lazarow PB (1981) Assay of peroxisomal beta-oxidation of fatty acids. Methods Enzymol 72:315–319
Qiu X, Brown K, Hirschey MD, Verdin E, Chen D (2010) Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12(6):662–667
Lehman TC, Hale DE, Bhala A, Thorpe C (1990) An acyl-coenzyme A dehydrogenase assay utilizing the ferricenium ion. Anal Biochem 186(2):280–284
Hwang HJ, Steidemann M, Dunivin TK, Rizzo M, LaPres JJ (2016) Data of enzymatic activities of the electron transport chain and ATP synthase complexes in mouse hepatoma cells following exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Data Brief 8:93–97
Thorsness PE, Koshland DE Jr (1987) Inactivation of isocitrate dehydrogenase by phosphorylation is mediated by the negative charge of the phosphate. J Biol Chem 262(22):10422–10425
Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, Ohe T, Namba M (1998) Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation 98(8):794–799
Jaffrey SR, Snyder SH (2001) The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE 2001(86):pl1
Kimura Y, Toyofuku Y, Koike S, Shibuya N, Nagahara N, Lefer D, Ogasawara Y, Kimura H (2015) Identification of H2S3 and H2S produced by 3-mercaptopyruvate sulfurtransferase in the brain. Sci Rep 5(1):14774. https://doi.org/10.1038/srep14774
Zhong X, Wang L, Wang Y, Dong S, Leng X, Jia J, Zhao Y, Li H, Zhang X, Xu C, Yang G, Wu L, Wang R, Lu F, Zhang W (2012) Exogenous hydrogen sulfide attenuates diabetic myocardial injury through cardiac mitochondrial protection. Mol Cell Biochem 371(1–2):187–198
Johnson AM, Olefsky JM (2013) The origins and drivers of insulin resistance. Cell 152(4):673–684
Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC (2010) Myocardial fatty acid metabolism in health and disease. Physiol Rev 90(1):207–258
Wagner GR, Hirschey MD (2014) Nonenzymatic protein acylation as a carbon stress regulated by sirtuin deacylases. Mol Cell 54(1):5–16
Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP (2002) SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci U S A 99(21):13653–13658
Garten A, Petzold S, Korner A, Imai S, Kiess W (2009) Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab 20(3):130–138
Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, Milbrandt J, Kiess W, Imai S (2007) Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab 6(5):363–375
Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, Kimura H (2009) A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal 11(2):205–214
Alrob OA, Sankaralingam S, Ma C, Wagg CS, Fillmore N, Jaswal JS, Sack MN, Lehner R, Gupta MP, Michelakis ED, Padwal RS, Johnstone DE, Sharma AM, Lopaschuk GD (2014) Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Cardiovasc Res 103(4):485–497
Duncan JG, Fong JL, Medeiros DM, Finck BN, Kelly DP (2007) Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation 115(7):909–917
Peterson LR, Saeed IM, McGill JB, Herrero P, Schechtman KB, Gunawardena R, Recklein CL, Coggan AR, DeMoss AJ, Dence CS, Gropler RJ (2012) Sex and type 2 diabetes: obesity-independent effects on left ventricular substrate metabolism and relaxation in humans. Obesity (Silver Spring) 20(4):802–810
Fillmore N, Mori J, Lopaschuk GD (2014) Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br J Pharmacol 171(8):2080–2090
Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV Jr, Alt FW, Kahn CR, Verdin E (2010) SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464(7285):121–125
Jing E, O'Neill BT, Rardin MJ, Kleinridders A, Ilkeyeva OR, Ussar S, Bain JR, Lee KY, Verdin EM, Newgard CB, Gibson BW, Kahn CR (2013) Sirt3 regulates metabolic flexibility of skeletal muscle through reversible enzymatic deacetylation. Diabetes 62(10):3404–3417
Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J, Li H, Xie L, Zhao W, Yao Y, Ning ZB, Zeng R, Xiong Y, Guan KL, Zhao S, Zhao GP (2010) Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science 327(5968):1004–1007
Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stancakova A, Goetzman E, Lam MM, Schwer B, Stevens RD, Muehlbauer MJ, Kakar S, Bass NM, Kuusisto J, Laakso M, Alt FW, Newgard CB, Farese RV Jr, Kahn CR, Verdin E (2011) SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell 44(2):177–190
Geng B, Chang L, Pan C, Qi Y, Zhao J, Pang Y, Du J, Tang C (2004) Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun 318(3):756–763
Su YW, Liang C, Jin HF, Tang XY, Han W, Chai LJ, Zhang CY, Geng B, Tang CS, JB D (2009) Hydrogen sulfide regulates cardiac function and structure in adriamycin-induced cardiomyopathy. Circ J 73(4):741–749
Bharathi SS, Zhang Y, Mohsen AW, Uppala R, Balasubramani M, Schreiber E, Uechi G, Beck ME, Rardin MJ, Vockley J, Verdin E, Gibson BW, Hirschey MD, Goetzman ES (2013) Sirtuin 3 (SIRT3) protein regulates long-chain acyl-CoA dehydrogenase by deacetylating conserved lysines near the active site. J Biol Chem 288(47):33837–33847
Vazquez EJ, Berthiaume JM, Kamath V, Achike O, Buchanan E, Montano MM, Chandler MP, Miyagi M, Rosca MG (2015) Mitochondrial complex I defect and increased fatty acid oxidation enhance protein lysine acetylation in the diabetic heart. Cardiovasc Res 107(4):453–465
Choi SE, Fu T, Seok S, Kim DH, Yu E, Lee KW, Kang Y, Li X, Kemper B, Kemper JK (2013) Elevated microRNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT. Aging Cell 12(6):1062–1072
Huang X, Gao Y, Qin J, Lu S (2014) The role of miR-34a in the hepatoprotective effect of hydrogen sulfide on ischemia/reperfusion injury in young and old rats. PLoS One 9(11):e113305. https://doi.org/10.1371/journal.pone.0113305
Acknowledgements
We thank Jingjie PTM BioLab Co.Ltd. (Hangzhou, China) for the mass spectrometry analysis.
Contribution statement
Zhang Weihua, Lu Fanghao and Sun Yu designed, developed, and performed the majority of the experiments. Zhiliang Tian, Lin Ning, Linxue Zhang, Shiyun Dong and Zhao Yajun performed mitochondrial enzyme activities analysis. Ren Huan, He Chen, Fan Yang, Jichao Wu, Yan Wang and Dechao Zhao provided additional bioinformatic data analysis. Gao Zhaopeng, Xiaojiao Sun, Miao Yu and Changqing Xu performed immunoprecipitation analysis. Zhang Weihua and Lu Fanghao supervised and managed the project and edited large sections of the manuscript. All authors contributed to writing and revising the manuscript.
To whom correspondence should be addressed Department of Pathophysiology, Harbin Medical university, Harbin, Heilongjiang Province 150086. Tel.:451-86674548; E-mail: zhangwh116@126.com; lufanghao1973@126.com. These authors contributed equally to this work.
Funding
This study was supported by the National Natural Science Foundation of China (81670344, 81370421, 81370330) and the Natural Science Foundation of Heilongjiang (No. D201070).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOC 2285 kb).
Rights and permissions
About this article
Cite this article
Sun, Y., Tian, Z., Liu, N. et al. Exogenous H2S switches cardiac energy substrate metabolism by regulating SIRT3 expression in db/db mice. J Mol Med 96, 281–299 (2018). https://doi.org/10.1007/s00109-017-1616-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1616-3