Abstract
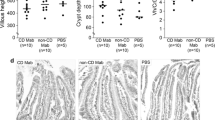
Typical features of celiac disease are small-bowel villus atrophy, crypt hyperplasia, and inflammation which develop gradually concomitant with ingestion of gluten. In addition, patients have anti-transglutaminase 2 (TG2) autoantibodies in their serum and tissues. The aim of this study was to establish whether celiac disease can be passively transferred to mice by serum or immunoglobulins. Serum aliquots or purified immunoglobulins (Ig) were intraperitoneally injected into Hsd:Athymic Nude-Foxn1nu mice for 8 or 27 days. As mice do not have proper IgA transport from peritoneum to blood, sera with a high content of IgG class anti-TG2 antibodies from untreated IgA-deficient celiac patients were used. Mouse sera were tested for celiac disease-specific autoantibodies, and several tissues were analyzed for autoantibody deposits targeted to TG2. Morphological assessment was made of the murine small intestinal mucosa. Injection of celiac disease patient sera or total IgG led to a significant delay in weight gain and mild diarrhea in a subset of mice. The mice injected with celiac patient sera or IgG had significantly decreased villus height crypt depth (Vh/CrD) ratios and celiac disease-specific autoantibody deposits targeted to TG2 in several tissues, including the small intestine. None of these features were observed in control mice. We conclude that administration of IgA-deficient celiac disease patient serum or total IgG induces both deterioration of the intestinal mucosa and clinical features of celiac disease in mice. The experimentally induced condition in the mice injected with patient serum or IgG resembles early developing celiac disease in humans.
Key message
-
Celiac disease patient sera or total IgG was injected into athymic mice.
-
A significant delay in weight gain and mild diarrhea was observed.
-
Mice evinced significantly decreased villus height crypt depth ratios.
-
Celiac disease-specific autoantibody deposits were present in several tissues.
-
The condition in mice resembles early stage celiac disease in humans.





Similar content being viewed by others
References
Kaukinen K, Lindfors K, Collin P, Koskinen O, Maki M (2010) Coeliac disease—a diagnostic and therapeutic challenge. Clin Chem Lab Med 48:1205–1216
Korponay-Szabo IR, Halttunen T, Szalai Z, Laurila K, Kiraly R, Kovacs JB, Fesus L, Maki M (2004) In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut 53:641–648
Salmi TT, Collin P, Jarvinen O, Haimila K, Partanen J, Laurila K, Korponay-Szabo IR, Huhtala H, Reunala T, Maki M et al (2006) Immunoglobulin a autoantibodies against transglutaminase 2 in the small intestinal mucosa predict forthcoming coeliac disease. Aliment Pharmacol Ther 24:541–552
Kurppa K, Collin P, Viljamaa M, Haimila K, Saavalainen P, Partanen J, Laurila K, Huhtala H, Paasikivi K, Maki M et al (2009) Diagnosing mild enteropathy celiac disease: a randomized, controlled clinical study. Gastroenterology 136:816–823
Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D (1997) Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med 3:797–801
Korponay-Szabo IR, Laurila K, Szondy Z, Halttunen T, Szalai Z, Dahlbom I, Rantala I, Kovacs JB, Fesus L, Maki M (2003) Missing endomysial and reticulin binding of coeliac antibodies in transglutaminase 2 knockout tissues. Gut 52:199–204
Marzari R, Sblattero D, Florian F, Tongiorgi E, Not T, Tommasini A, Ventura A, Bradbury A (2001) Molecular dissection of the tissue transglutaminase autoantibody response in celiac disease. J Immunol 166:4170–4176
Koskinen O, Collin P, Korponay-Szabo I, Salmi T, Iltanen S, Haimila K, Partanen J, Maki M, Kaukinen K (2008) Gluten-dependent small bowel mucosal transglutaminase 2-specific IgA deposits in overt and mild enteropathy coeliac disease. J Pediatr Gastroenterol Nutr 47:436–442
Di Niro R, Mesin L, Zheng NY, Stamnaes J, Morrissey M, Lee JH, Huang M, Iversen R, du Pre MF, Qiao SW et al (2012) High abundance of plasma cells secreting transglutaminase 2-specific IgA autoantibodies with limited somatic hypermutation in celiac disease intestinal lesions. Nat Med 18:441–445
Kaukinen K, Peraaho M, Collin P, Partanen J, Woolley N, Kaartinen T, Nuutinen T, Halttunen T, Maki M, Korponay-Szabo I (2005) Small-bowel mucosal transglutaminase 2-specific IgA deposits in coeliac disease without villous atrophy: a prospective and randomized clinical study. Scand J Gastroenterol 40:564–572
Tosco A, Salvati VM, Auricchio R, Maglio M, Borrelli M, Coruzzo A, Paparo F, Boffardi M, Esposito A, D’Adamo G et al (2011) Natural history of potential celiac disease in children. Clin Gastroenterol Hepatol 9:320–325, quiz e336
Hadjivassiliou M, Maki M, Sanders DS, Williamson CA, Grunewald RA, Woodroofe NM, Korponay-Szabo IR (2006) Autoantibody targeting of brain and intestinal transglutaminase in gluten ataxia. Neurology 66:373–377
Holmes GK (2002) Screening for coeliac disease in type 1 diabetes. Arch Dis Child 87:495–498
Iltanen S, Collin P, Korpela M, Holm K, Partanen J, Polvi A, Maki M (1999) Celiac disease and markers of celiac disease latency in patients with primary Sjogren’s syndrome. Am J Gastroenterol 94:1042–1046
Korponay-Szabo IR, Dahlbom I, Laurila K, Koskinen S, Woolley N, Partanen J, Kovacs JB, Maki M, Hansson T (2003) Elevation of IgG antibodies against tissue transglutaminase as a diagnostic tool for coeliac disease in selective IgA deficiency. Gut 52:1567–1571
Borrelli M, Maglio M, Agnese M, Paparo F, Gentile S, Colicchio B, Tosco A, Auricchio R, Troncone R (2010) High density of intraepithelial gammadelta lymphocytes and deposits of immunoglobulin (Ig) M anti-tissue transglutaminase antibodies in the jejunum of coeliac patients with IgA deficiency. Clin Exp Immunol 160:199–206
Cataldo F, Marino V, Ventura A, Bottaro G, Corazza GR (1998) Prevalence and clinical features of selective immunoglobulin a deficiency in coeliac disease: an Italian multicentre study. Italian Society of paediatric gastroenterology and hepatology (SIGEP) and “club del tenue” working groups on coeliac disease. Gut 42:362–365
Kalliokoski S, Sulic AM, Korponay-Szabo IR, Szondy Z, Frias R, Perez MA, Martucciello S, Roivainen A, Pelliniemi LJ, Esposito C et al (2013) Celiac disease-specific TG2-targeted autoantibodies inhibit angiogenesis ex vivo and in vivo in mice by interfering with endothelial cell dynamics. PLoS One 8:e65887
Lindfors K, Maki M, Kaukinen K (2010) Transglutaminase 2-targeted autoantibodies in celiac disease: pathogenetic players in addition to diagnostic tools? Autoimmun Rev 9:744–749
Barone MV, Caputo I, Ribecco MT, Maglio M, Marzari R, Sblattero D, Troncone R, Auricchio S, Esposito C (2007) Humoral immune response to tissue transglutaminase is related to epithelial cell proliferation in celiac disease. Gastroenterology 132:1245–1253
Halttunen T, Maki M (1999) Serum immunoglobulin A from patients with celiac disease inhibits human T84 intestinal crypt epithelial cell differentiation. Gastroenterology 116:566–572
Cervio E, Volta U, Verri M, Boschi F, Pastoris O, Granito A, Barbara G, Parisi C, Felicani C, Tonini M et al (2007) Sera of patients with celiac disease and neurologic disorders evoke a mitochondrial-dependent apoptosis in vitro. Gastroenterology 133:195–206
Harrington WJ, Minnich V, Hollingsworth JW, Moore CV (1951) Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. J Lab Clin Med 38:1–10
Monteiro RC, Van De Winkel JG (2003) IgA Fc receptors. Annu Rev Immunol 21:177–204
Bogers WM, Gorter A, Stuurman ME, Van Es LA, Daha MR (1989) Clearance kinetics and tissue distribution of aggregated human serum IgA in rats. Immunology 67:274–280
Marsh MN (1992) Gluten, major histocompatibility complex, and the small intestine. a molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 102:330–354
Taavela J, Koskinen O, Huhtala H, Lahdeaho ML, Popp A, Laurila K, Collin P, Kaukinen K, Kurppa K, Maki M (2013) Validation of morphometric analyses of small-intestinal biopsy readouts in celiac disease. PLoS One 8:e76163
Upchurch HF, Conway E, Patterson MK Jr, Maxwell MD (1991) Localization of cellular transglutaminase on the extracellular matrix after wounding: characteristics of the matrix bound enzyme. J Cell Physiol 149:375–382
Korponay-Szabo IR, Sulkanen S, Halttunen T, Maurano F, Rossi M, Mazzarella G, Laurila K, Troncone R, Maki M (2000) Tissue transglutaminase is the target in both rodent and primate tissues for celiac disease-specific autoantibodies. J Pediatr Gastroenterol Nutr 31:520–527
Kaukinen K, Maki M, Partanen J, Sievanen H, Collin P (2001) Celiac disease without villous atrophy: revision of criteria called for. Dig Dis Sci 46:879–887
Di Niro R, Sblattero D, Florian F, Stebel M, Zentilin L, Giacca M, Villanacci V, Galletti A, Not T, Ventura A et al (2008) Anti-idiotypic response in mice expressing human autoantibodies. Mol Immunol 45:1782–1791
Boscolo S, Sarich A, Lorenzon A, Passoni M, Rui V, Stebel M, Sblattero D, Marzari R, Hadjivassiliou M, Tongiorgi E (2007) Gluten ataxia: passive transfer in a mouse model. Ann N Y Acad Sci 1107:319–328
Boscolo S, Lorenzon A, Sblattero D, Florian F, Stebel M, Marzari R, Not T, Aeschlimann D, Ventura A, Hadjivassiliou M et al (2010) Anti transglutaminase antibodies cause ataxia in mice. PLoS One 5:e9698
Toyka KV, Drachman DB, Griffin DE, Pestronk A, Winkelstein JA, Fishbeck KH, Kao I (1977) Myasthenia gravis. study of humoral immune mechanisms by passive transfer to mice. N Engl J Med 296:125–131
Petkova SB, Konstantinov KN, Sproule TJ, Lyons BL, Awwami MA, Roopenian DC (2006) Human antibodies induce arthritis in mice deficient in the low-affinity inhibitory IgG receptor Fc gamma RIIB. J Exp Med 203:275–280
Anhalt GJ, Labib RS, Voorhees JJ, Beals TF, Diaz LA (1982) Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N Engl J Med 306:1189–1196
Roscoe JT, Diaz L, Sampaio SA, Castro RM, Labib RS, Takahashi Y, Patel H, Anhalt GJ (1985) Brazilian pemphigus foliaceus autoantibodies are pathogenic to BALB/c mice by passive transfer. J Invest Dermatol 85:538–541
Katzav A, Arango MT, Kivity S, Tanaka S, Givaty G, Agmon-Levin N, Honda M, Anaya JM, Chapman J, Shoenfeld Y (2013) Passive transfer of narcolepsy: anti-TRIB2 autoantibody positive patient IgG causes hypothalamic orexin neuron loss and sleep attacks in mice. J Autoimmun 45:24–30
Acknowledgments
This work was supported by grants to the Celiac Disease Study Group from the University of Tampere, the Academy of Finland, the Sigrid Juselius Foundation, the Competitive State Research Financing of the Expert Area of Tampere University Hospital (grants 9R034 and 9R018) and Seinäjoki Central Hospital (VTR16), the Research Fund of the Finnish Coeliac Society, the Finnish Cultural Foundation, the Hungarian Scientific Research Fund (OTKA K101788), TÁMOP 4.2.2.A-11/1/KONV-2012-0023 VED-ELEM, and the European Commission (contract number PIA-GA-2010-251506).
Conflict of interest
The authors declare no conflict of interests related to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalliokoski, S., Caja, S., Frias, R. et al. Injection of celiac disease patient sera or immunoglobulins to mice reproduces a condition mimicking early developing celiac disease. J Mol Med 93, 51–62 (2015). https://doi.org/10.1007/s00109-014-1204-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-014-1204-8