Abstract
Purpose
To optimize and validate a current (NRG [a newly constituted National Clinical Trials Network group through National Surgical Adjuvant Breast and Bowel Project [NSABP], the Radiation Therapy Oncology Group [RTOG] and the Gynecologic Oncology Group (GOG)]) nomogram for glioblastoma patients as part of continuous validation.
Methods
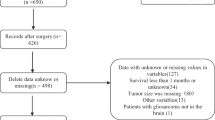
We identified patients newly diagnosed with glioblastoma who were treated with temozolomide-based chemoradiotherapy between 2006 and 2016 at three large-volume hospitals. The extent of resection was determined via postoperative MRI. The discrimination and calibration abilities of the prediction algorithm were assessed; if additional factors were identified as independent prognostic factors, updated models were developed using the data from two hospitals and were externally validated using the third hospital. Models were internally validated using cross-validation and bootstrapping.
Results
A total of 837 patients met the eligibility criteria. The median overall survival (OS) was 20.0 (95% CI 18.5–21.5) months. The original nomogram was able to estimate the 6‑, 12-, and 24-month OS probabilities, but it slightly underestimated the OS values. In multivariable Cox regression analysis, MRI-defined total resection had a greater impact on OS than that shown by the original nomogram, and two additional factors—IDH1 mutation and tumor contacting subventricular zone—were newly identified as independent prognostic values. An updated nomogram incorporating these new variables outperformed the original nomogram (C-index at 6, 12, 24, and 36 months: 0.728, 0.688, 0.688, and 0.685, respectively) and was well calibrated. External validation using an independent cohort showed C‑indices of 0.787, 0.751, 0.719, and 0.702 at 6, 12, 24, and 36 months, respectively, and was well calibrated.
Conclusion
An updated and validated nomogram incorporating the contemporary parameters can estimate individual survival outcomes in patients with glioblastoma with better accuracy.
Zusammenfassung
Ziel der Arbeit
Ziel der vorliegenden Arbeit war die Optimierung und Validierung eines aktuellen NRG-Nomogramms für Glioblastompatienten im Rahmen einer kontinuierlichen Validierung.
Methoden
Die Autoren identifizierten Patienten mit neu diagnostiziertem Glioblastom, die zwischen 2006 und 2016 in 3 großen Krankenhäusern mit einer temozolomidbasierten Radiochemotherapie behandelt wurden. Das Ausmaß der Resektion wurde mittels postoperativer Magnetresonanztomographie (MRT) bestimmt. Die Diskriminierung- und Kalibrierungsfähigkeit des Prognosealgorithmus wurden bewertet. Unter Einbeziehung zusätzlicher Faktoren, die als unabhängige prognostische Faktoren identifiziert wurden, entwickelten die Autoren aktualisierte Modelle unter Verwendung der Daten von 2 Zentren. Diese wurden mit Daten aus dem dritten Zentrum extern validiert. Die Modelle wurden mithilfe der Kreuzvalidierung und Bootstrapping intern bestätigt.
Ergebnisse
Insgesamt 837 Patienten erfüllten die Einschlusskriterien. Das mediane Gesamtüberleben (OS) betrug 20,0 (95%-Konfidenzintervall, 95%-KI: 18,5–21,5) Monate. Mit dem ursprünglichen Nomogramm konnten die OS-Wahrscheinlichkeiten für 6, 12 und 24 Monate geschätzt werden, die OS-Werte wurden jedoch geringfügig unterschätzt. In der multivariablen Cox-Regressionsanalyse wirkte sich das MRT-definierte Ausmaß der Resektion stärker auf das OS als im ursprünglichen Nomogramm aus. Es wurden 2 zusätzliche Faktoren, eine IDH1-Mutation und der Kontakt des Tumors zur subventrikulären Zone, neu als unabhängige prognostische Werte definiert. Ein aktualisiertes Nomogramm, das diese neuen Variablen enthält, ist gut kalibriert und war dem ursprünglichen Nomogramm (C-Index bei 6, 12, 24 und 36 Monaten: 0,728; 0,688; 0,688 und 0,685) überlegen. Die externe Validierung mit einer unabhängigen Kohorte ist gut kalibriert und ergab C‑Indizes von 0,787; 0,751; 0,719 und 0,702 bei jeweils 6, 12, 24 und 36 Monaten.
Schlussfolgerungen
Mit einem aktualisierten und validierten Nomogramm, welches aktualisierte Parameter berücksichtigt, ist es möglich, die individuelle Überlebensfähigkeit von Patienten mit einem Glioblastom mit größerer Genauigkeit abzuschätzen.




Similar content being viewed by others
References
Bell EH, Pugh SL, McElroy JP et al (2017) Molecular-based recursive partitioning analysis model for Glioblastoma in the Temozolomide era: a correlative analysis based on NRG oncology RTOG 0525. JAMA Oncol 3:784–792
Chen J, McKay RM, Parada LF (2012) Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell 149:36–47
Choi SH, Kim JW, Chang JS et al (2017) Impact of including Peritumoral edema in radiotherapy target volume on patterns of failure in Glioblastoma following Temozolomide-based Chemoradiotherapy. Sci Rep 7:42148
Curran WJ Jr., Scott CB, Horton J et al (1993) Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst 85:704–710
Elicin O, Inac E, Uzel EK et al (2014) Relationship between survival and increased radiation dose to subventricular zone in glioblastoma is controversial. J Neurooncol 118:413–419
GBD Mortality and Causes of Death Collaborators (2015) Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385:117–171
Gittleman H, Lim D, Kattan MW et al (2017) An independently validated nomogram for individualized estimation of survival among patients with newly diagnosed glioblastoma: NRG Oncology RTOG 0525 and 0825. Neuro-Oncology 19:669–677
Iasonos A, Schrag D, Raj GV et al (2008) How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 26:1364–1370
Ingrisch M, Schneider MJ, Norenberg D et al (2017) Radiomic analysis reveals prognostic information in T1-weighted baseline magnetic resonance imaging in patients with Glioblastoma. Invest Radiol 52:360–366
Jafri NF, Clarke JL, Weinberg V et al (2013) Relationship of glioblastoma multiforme to the subventricular zone is associated with survival. Neuro-Oncology 15:91–96
Kickingereder P, Bonekamp D, Nowosielski M et al (2016) Radiogenomics of Glioblastoma: machine learning-based classification of molecular characteristics by using Multiparametric and Multiregional MR imaging features. Radiology 281:907–918
Kim YS, Kim SH, Cho J et al (2012) MGMT gene promoter methylation as a potent prognostic factor in glioblastoma treated with temozolomide-based chemoradiotherapy: a single-institution study. Int J Radiat Oncol Biol Phys 84:661–667
Lacroix M, Abi-Said D, Fourney DR et al (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198
Lacroix M, Toms SA (2014) Maximum safe resection of glioblastoma multiforme. J Clin Oncol 32:727–728
Lai A, Tran A, Nghiemphu PL et al (2011) Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol 29:142–148
Lee J, Cho J, Chang JH et al (2016) Re-irradiation for recurrent gliomas: treatment outcomes and prognostic factors. Yonsei Med J 57:824–830
Li J, Wang M, Won M et al (2011) Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys 81:623–630
Li YM, Suki D, Hess K et al (2016) The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg 124:977–988
Liang TH, Kuo SH, Wang CW et al (2016) Adverse prognosis and distinct progression patterns after concurrent chemoradiotherapy for glioblastoma with synchronous subventricular zone and corpus callosum invasion. Radiother Oncol 118:16–23
Lim DA, Cha S, Mayo MC et al (2007) Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro-Oncology 9:424–429
Marko NF, Weil RJ, Schroeder JL et al (2014) Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol 32:774–782
Mistry AM, Dewan MC, White-Dzuro GA et al (2017) Decreased survival in glioblastomas is specific to contact with the ventricular-subventricular zone, not subgranular zone or corpus callosum. J Neurooncol 132:341–349
Molenaar RJ, Verbaan D, Lamba S et al (2014) The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro-Oncology 16:1263–1273
Molitoris JK, Rao YJ, Patel RA et al (2017) Multi-institutional external validation of a novel glioblastoma prognostic nomogram incorporating MGMT methylation. J Neurooncol 134:331–338
Ostrom QT, Gittleman H, Liao P et al (2017) CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-Oncology 19:v1–v88
Paravati AJ, Heron DE, Landsittel D et al (2011) Radiotherapy and temozolomide for newly diagnosed glioblastoma and anaplastic astrocytoma: Validation of Radiation Therapy Oncology Group-Recursive Partitioning Analysis in the IMRT and temozolomide era. J Neurooncol 104:339–349
Perry JR, Laperriere N, O’Callaghan CJ et al (2017) Short-course radiation plus Temozolomide in elderly patients with Glioblastoma. N Engl J Med 376:1027–1037
Roh TH, Park HH, Kang SG et al (2017) Long-term outcomes of concomitant chemoradiotherapy with temozolomide for newly diagnosed glioblastoma patients: a single-center analysis. Medicine (Baltimore) 96:e7422
Scott CB, Scarantino C, Urtasun R et al (1998) Validation and predictive power of Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis classes for malignant glioma patients: a report using RTOG 90-06. Int J Radiat Oncol Biol Phys 40:51–55
Scott JG, Bauchet L, Fraum TJ et al (2012) Recursive partitioning analysis of prognostic factors for glioblastoma patients aged 70 years or older. Cancer 118:5595–5600
Sinnaeve J, Mobley BC, Ihrie RA (2018) Space invaders: brain tumor exploitation of the stem cell niche. Am J Pathol 188:29–38
Smith AW, Mehta MP, Wernicke AG (2016) Neural stem cells, the subventricular zone and radiotherapy: implications for treating glioblastoma. J Neurooncol 128:207–216
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5‑year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Wee CW, Kim E, Kim N et al (2017) Glioblastoma Novel recursive partitioning analysis classification for newly diagnosed glioblastoma: a multi-institutional study highlighting the MGMT promoter methylation and IDH1 gene mutation status. Radiother Oncol 123:106–111
Acknowledgements
The authors would like to thank Franziska Walter (LMU University Hospital, 81377, Munich, Germany) for her help with the German abstract of this manuscript.
Author information
Authors and Affiliations
Contributions
N.K., J.S.C, C.W.W, and I.A.K analyzed the data. J.H.C, S.H.K., S.K., E.H.K., H.I.Y., J.W.K., C.H., J.C., E.K., T.M.K., Y.J.K., C.P., J.W.K., C.K., S.H.C, J.H.K., S.P., G.C, and S.L. provided clinical samples, reviewed and provided insight to the manuscript. H.S.L provided the statistical analysis N.K., J.S.C., I.H.K., and C.O.S designed the study and supervised the overall project. N.K. and J.S.C. wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
N. Kim, J.S. Chang, C.W. Wee, I.A. Kim, J.H. Chang, H.S. Lee, S.H. Kim, S.-G. Kang, E.H. Kim, H.I. Yoon, J.W. Kim, C.-K. Hong, J. Cho, E. Kim, T.M. Kim, Y.J. Kim, C.-K. Park, J.W. Kim, C.-Y. Kim, S.H. Choi, J.H. Kim, S.-H. Park, G. Choe, S.-T. Lee, I.H. Kim, and C.-O. Suh declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was waived due to the retrospective nature of this study.
Additional information
Presented at the 15th Meeting of the Asian Society for Neuro-Oncology, October 2017, Osaka, Japan. Presented in poster form at the 60th Annual Meeting of the American Society for Radiation Oncology, October 2018, San Antonio, TX, USA
Caption Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Kim, N., Chang, J.S., Wee, C.W. et al. Validation and optimization of a web-based nomogram for predicting survival of patients with newly diagnosed glioblastoma. Strahlenther Onkol 196, 58–69 (2020). https://doi.org/10.1007/s00066-019-01512-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-019-01512-y