Abstract
Background
As an adjuvant therapeutic strategy in advanced gastric cancer, both adjuvant chemotherapy (CTx) and postoperative radiochemotherapy (RCTx) can be considered. Both approaches have been shown to improve overall survival compared to resection alone. Several prospective randomized trials have compared the two postoperative concepts.
Methods
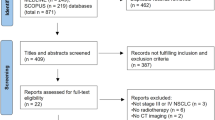
We performed a literature search to identify prospective randomized trials which compared adjuvant chemotherapy to adjuvant radiochemotherapy in patients with advanced gastric cancer. As effect sizes, we extracted hazard ratios (HR) as well as event rates from the included trials for the endpoints overall survival, disease-free survival and locoregional control.
Results
We identified seven studies that enrolled 1807 patients overall. Combined radiochemotherapy showed no significant improvement of overall survival in comparison to chemotherapy alone (HR = 0.93; 95%CI: 0.82–1.06; p = 0.28). For disease-free survival (HR = 0.86; 95%CI: 0.76–0.98; p = 0.023) and locoregional control (odds ratio [OR] = 0.56; 95%CI: 0.42–0.75; p = <0.001) we detected significant advantages from the addition of radiation to chemotherapy. A subgroup analysis demonstrated an improvement in survival when the radiochemotherapy protocol was not de-intensified.
Conclusions
Adjuvant chemotherapy or radiochemotherapy demonstrate similar oncologic efficacy and therapy-associated toxicity. Individual patient characteristics should therefore determine the therapeutic approach in a multidisciplinary discussion. Irradiation added to standard-dose chemotherapy possibly results in a survival benefit.
Zusammenfassung
Hintergrund
Als adjuvante Therapiestrategie beim fortgeschrittenen Magenkarzinom kommen sowohl die adjuvante Chemotherapie (CTx) als auch die postoperative Radiochemotherapie (RCTx) in Frage. Für beide Ansätze konnte eine Verbesserung des Gesamtüberlebens im Vergleich zur alleinigen Operation gezeigt werden. Mehrere prospektive, randomisierte Studien haben die beiden postoperativen Konzepte miteinander verglichen.
Methoden
Wir führten eine Literaturrecherche zur Identifikation von prospektiven, randomisierten Studien durch, die bei Patienten mit einem fortgeschrittenen Magenkarzinom eine CTx mit einer RCTx verglichen. Als Effektschätzer wurden die „Hazard Ratios“ (HR) sowie die Ereignisraten aus den eingeschlossenen Studien für die Endpunkte Gesamtüberleben, krankheitsfreies Überleben und lokoregionäre Kontrolle extrahiert.
Ergebnisse
Es wurden sieben Studien identifiziert, die insgesamt 1807 Patienten einschlossen. Eine kombinierte RCTx zeigte keine signifikante Verbesserung des Gesamtüberlebens im Vergleich der alleinigen CTx (HR = 0,93; 95 %-Konfidenzintervall [KI] 0,82–1,06; p = 0,28). Hinsichtlich des krankheitsfreien Überlebens (HR = 0,86; 95 %-KI 0,76–0,98; p = 0,023) und lokaler Tumorkontrolle (Odds Ratio [OR] = 0,56; 95 %-KI 0,42–0,75; p = <0,001) konnten klare statistische Vorteile der Addition von Strahlentherapie zur Chemotherapie detektiert werden. Eine Subgruppenanalyse zeigte einen Überlebensvorteil, wenn die RCTx nicht deintensiviert appliziert wurde.
Schlussfolgerung
Adjuvante CTx und RCTx zeigen ähnliche onkologische Effektivität und therapieassoziierte Toxizität, sodass die individuelle Patientencharakteristik die Therapieentscheidung im Rahmen einer multidisziplinären Diskussion bestimmen sollte. Möglicherweise resultiert aus der Strahlentherapie zusammen mit der volldosierten Chemotherapie ein Überlebensvorteil.





Similar content being viewed by others
References
Bamias A, Karina M, Papakostas P et al (2010) A randomized phase III study of adjuvant platinum/docetaxel chemotherapy with or without radiation therapy in patients with gastric cancer. Cancer Chemother Pharmacol 65:1009–1021
van der Kaaij RT, van Kessel JP, van Dieren JM et al (2018) Outcomes after prophylactic gastrectomy for hereditary diffuse gastric cancer. Br J Surg 105:e176–e82
van der Post RS, van Dieren J, Grelack A et al (2018) Outcomes of screening gastroscopy in first-degree relatives of patients fulfilling hereditary diffuse gastric cancer criteria. Gastrointest Endosc 87:397–404.e2
Claassen YHM, van Amelsfoort RM, Hartgrink HH, Dikken JL, de Steur WO, van Sandick JW, van Grieken NCT, Cats A, Boot H, Trip AK, Jansen EPM, Kranenbarg EM, Braak JPBM, Putter H, van Berge Henegouwen MI, Verheij M, van de Velde CJH (2018) Effect of hospital volume with respect to performing gastric cancer resection on recurrence and survival: results from the CRITICS trial. Ann Surg. https://doi.org/10.1097/sla.0000000000002940
Deng J, Liang H, Sun D, Pan Y, Liu Y, Wang D (2011) Extended lymphadenectomy improvement of overall survival of gastric cancer patients with perigastric node metastasis. Langenbecks Arch Surg 396:615–623
Deng J, Liang H, Wang D (2011) The feasibility of N stage of the 7th edition TNM for gastric cancer. Ann Surg Oncol 18:1805–1806
Deng J, Liang H, Wang D, Sun D, Pan Y, Liu Y (2011) Investigation of the recurrence patterns of gastric cancer following a curative resection. Surg Today 41:210–215
Dreznik A, Purim O, Idelevich E et al (2012) Gastric cancer: biology and clinical manifestations in Israel. J Surg Oncol 105:316–322
Smyth EC, Verheij M, Allum W et al (2016) Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 27:v38–v49
Cunningham D, Allum WH, Stenning SP et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11–20
Smalley SR, Benedetti JK, Haller DG et al (2012) Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 30:2327–2333
Macdonald JS, Smalley SR, Benedetti J et al (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345:725–730
Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17:2815–2834
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8:16
Doi SA, Barendregt JJ, Khan S, Thalib L, Williams GM (2015) Advances in the meta-analysis of heterogeneous clinical trials I: The inverse variance heterogeneity model. Contemp Clin Trials 45:130–138
Park SH, Sohn TS, Lee J et al (2015) Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: final report of the Adjuvant Chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J Clin Oncol 33:3130–3136
Yu C, Yu R, Zhu W, Song Y, Li T (2012) Intensity-modulated radiotherapy combined with chemotherapy for the treatment of gastric cancer patients after standard D1/D2 surgery. J Cancer Res Clin Oncol 138:255–259
Kwon HC, Kim MC, Kim KH et al (2010) Adjuvant chemoradiation versus chemotherapy in completely resected advanced gastric cancer with D2 nodal dissection. Asia Pac J Clin Oncol 6:278–285
Kim TH, Park SR, Ryu KW et al (2012) Phase 3 trial of postoperative chemotherapy alone versus chemoradiation therapy in stage III–IV gastric cancer treated with R0 gastrectomy and D2 lymph node dissection. Int J Radiat Oncol Biol Phys 84:e585–e592
Cats A, Jansen EPM, van Grieken NCT et al (2018) Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol 19:616–628
Zhu WG, Xua DF, Pu J et al (2012) A randomized, controlled, multicenter study comparing intensity-modulated radiotherapy plus concurrent chemotherapy with chemotherapy alone in gastric cancer patients with D2 resection. Radiother Oncol 104:361–366
Fuchs CS, Niedzwiecki D, Mamon HJ et al (2017) Adjuvant chemoradiotherapy with epirubicin, cisplatin, and fluorouracil compared with adjuvant chemoradiotherapy with fluorouracil and leucovorin after curative resection of gastric cancer: results from CALGB 80101 (alliance). J Clin Oncol 35:3671–3677
Nam H, Lim DH, Kim S et al (2008) A new suggestion for the radiation target volume after a subtotal gastrectomy in patients with stomach cancer. Int J Radiat Oncol Biol Phys 71:448–455
Yang J, Liu J, Gao S et al (2018) Use of simultaneous radiation boost achieves high treatment response rate in patients with metastatic gastric cancer. J Cancer Res Ther 14:36–39
Al-Batran SE, Ajani JA (2010) Impact of chemotherapy on quality of life in patients with metastatic esophagogastric cancer. Cancer 116:2511–2518
Kassam Z, Mackay H, Buckley CA et al (2010) Evaluating the impact on quality of life of chemoradiation in gastric cancer. Curr Oncol 17:77–84
Goody RB, Mackay H, Pitcher B et al (2016) Prospective evaluation of quality of life (QOL) during a phase I/II study of adjuvant chemotherapy with image-guided high-precision radiotherapy for completely resected gastric cancer. J Clin Oncol 34:164
Amelsfoort RV, Walraven I, Jansen EP et al (2018) Quality of life in the CRITICS study, a multicenter randomized phase III trial of neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy in resectable gastric cancer. J Clin Oncol 36:4060
Dikken JL, Jansen EP, Cats A et al (2010) Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol 28:2430–2436
Stiekema J, Trip AK, Jansen EP et al (2015) Does adjuvant chemoradiotherapy improve the prognosis of gastric cancer after an r1 resection? Results from a dutch cohort study. Ann Surg Oncol 22:581–588
Fiorica F, Trovo M, Ottaiano A et al (2018) Can the addition of radiotherapy postoperatively increase clinical outcome of patients with gastric cancer? A systematic review of the literature and meta-analysis. Oncotarget 9:10734–10744
Leong T, Smithers BM, Haustermans K et al (2017) TOPGEAR: a randomized, phase III trial of perioperative ECF chemotherapy with or without preoperative chemoradiation for resectable gastric cancer: interim results from an international, intergroup trial of the AGITG, TROG, EORTC and CCTG. Ann Surg Oncol 24:2252–2258
Ajani JA, Winter K, Okawara GS et al (2006) Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol 24:3953–3958
Chakravarty T, Crane CH, Ajani JA et al (2012) Intensity-modulated radiation therapy with concurrent chemotherapy as preoperative treatment for localized gastric adenocarcinoma. Int J Radiat Oncol Biol Phys 83:581–586
Al-Batran S‑E, Homann N, Schmalenberg H et al (2017) Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): A multicenter, randomized phase 3 trial. J Clin Oncol 35:4004
Lee J, Lim DH, Kim S et al (2012) Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 30:268–273
Author information
Authors and Affiliations
Contributions
C. Matuschek and J. Haussmann contributed equally to the manuscript. E. Bölke and W. Budach had the idea, coordinated the work, and wrote parts of the manuscript. E. Bölke did the literature research, prepared the data for analysis, and wrote parts of the manuscript. K. Kammers and J. Haussmann did the statistical analysis. C. Matuschek, J. Haussmann, K. Orth, and M. Peiper wrote parts of the manuscript. J. Haussmann contributed significantly to the discussion on the interpretation of the results. C. Matuschek, J. Haussmann, and P.A. Gerber prepared the figures and tables and wrote parts of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
C. Matuschek, J. Haussmann, E. Bölke, B. Tamaskovics, F.-J. Djiepmo Njanang, K. Orth, M. Peiper, P.A. Gerber, B. Anooshar, K. Kammers, and W. Budach declare that they have no competing interests.
Ethical standards
The authors did not conduct any studies with human or animal participants for this article. As for other studies cited in this article, information on ethical guidelines may be found in the respective sources.
Additional information
This work was presented in parts at the Annual ASCO (American Society of Clinical Oncology) meeting in Chicago 2018.
Christiane Matuschek and Jan Haussmann contributed equally to this work.
Rights and permissions
About this article
Cite this article
Matuschek, C., Haussmann, J., Bölke, E. et al. Adjuvant radiochemotherapy vs. chemotherapy alone in gastric cancer: a meta-analysis. Strahlenther Onkol 195, 695–706 (2019). https://doi.org/10.1007/s00066-019-01431-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-019-01431-y
Keywords
- Advanced gastric cancer
- Randomized trials
- Disease free survival
- Local regional control
- Literature search