Abstract
Aim
It is recognized that stereotactic body radiotherapy (SBRT) for centrally located lung metastases is affected by higher rates of severe toxicity. In the present study, we report the clinical outcomes following a novel intensity-modulated radiotherapy prescription dose, termed simultaneous integrated protection (SIP), for nearby organs at risk (OARs).
Materials and methods
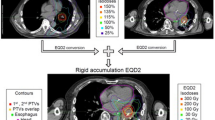
The prescribed total doses of SBRT were 70 Gy in 10 fractions and 60 Gy in 8 fractions. For ultra-centrally located lesions, a dose of 60 Gy in 10 fractions was delivered. The main planning instructions were: (1) to remain within the limits of the given dose constraints for an OAR; (2) to make use of the maximum possible dose to the OARs to minimize dose inhomogeneity for the Planning Target Volume (PTV). SBRT-related toxicity was prospectively assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.0. The primary clinical endpoint was the SBRT-related toxicity. Secondary endpoint was local control.
Results
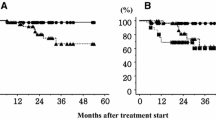
Forty patients affected by a single central malignancy were analyzed. The median follow-up was 20 months (range, 6–58 months). Acute and late clinical pulmonary toxicity ≥grade 2 was recorded in 2 out of 40 patients (5%) and 3 out of 40 patients (7%), respectively. No patient experienced cardiac toxicity. No narrowing or stenosis of any airway or vessel was registered. One-year local control rate was 91%. The median time to local progression was 13 months (range, 6–46 months).
Conclusion
SBRT using a PTV-SIP approach for single central lung metastases achieved low SBRT-related toxicity with acceptable local control.
Zusammenfassung
Ziel
Die extrakranielle Körperstereotaxie (SBRT) von zentral gelegenen Lungentumoren weist höhergradige Toxizitäten auf. In der vorliegenden Studie berichten wir über die klinischen Ergebnisse einer neuen intensitätsmodulierten Technik, welche zur Reduktion der Toxizität eine simultan integrierte Protektion (SIP) von Risikoorganen (OARs) anwendet.
Material und Methoden
Die Verschreibungsdosis der SBRT war 70 Gy in 10 Fraktionen und 60 Gy in 8 Fraktionen. Bei sehr zentral gelegenen Läsionen wurde eine Dosis von 60 Gy in 10 Fraktionen appliziert. Die wichtigsten Punkte bei der Bestrahlungsplanung waren folgende: (1) innerhalb der Toleranzgrenzen der OAR zu bleiben; (2) die maximale Dosis eines OARs auszureizen, um die Dosisinhomogenität für das PTV zu minimieren. Die therapiebezogene Toxizität wurde prospektiv nach den Common Terminology Criteria for Adverse Events (CTCAE) v4.0 erhoben. Der primäre klinische Endpunkt war die SBRT-bezogene Toxizität. Sekundärer Endpunkt war die lokale Kontrolle.
Ergebnisse
Insgesamt wurden 40 Patienten an einem singulären zentralen Lungentumor behandelt. Das mediane Follow-up betrug 20 Monate (Spanne 6–58 Monate). Akute pulmonale Nebenwirkungen ≥Grad 2 wurden bei 2 von 40 Patienten (5%) festgestellt und pulmonale Spätnebenwirkungen (≥Grad 2) bei 3 von 40 Patienten (7%). Kein Patient wies eine kardiale Toxizität auf. Es wurde keine Verengung oder Stenose der Atemwege oder Gefäße festgestellt. Die lokale 1‑Jahres-Kontrollrate lag bei 91%. Die mediane Zeit bis zum lokalen Progress betrug 13 Monate (Spanne 6–46 Monate).
Schlussfolgerung
Eine SBRT unter Verwendung eines PTV-SIP-Ansatzes für zentrale Lungenmalignome ermöglicht eine akzeptable lokale Tumorkontrolle bei niedriger therapieassoziierter Toxizität.


Similar content being viewed by others
References
Alongi F, Arcangeli S, Filippi AR, Ricardi U, Scorsetti M (2012) Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist 17(8):1100–1107
Borm KJ, Oechsner M, Schiller K, Peeken JC, Dapper H, Münch S et al (2018) Prognostic factors in stereotactic body radiotherapy of lung metastases. Strahlenther Onkol. https://doi.org/10.1007/s00066-018-1335-x
Schanne DH, Nestle U, Allgäuer M, Andratschke N, Appold S, Dieckmann U et al (2015) Stereotactic body radiotherapy for centrally located stage I NSCLC: a multicenter analysis. Strahlenther Onkol 191(2):125–132
Mazzola R, Fersino S, Ferrera G, Targher G, Figlia V, Triggiani L et al (2018) Stereotactic body radiotherapy for lung oligometastases impacts on systemic treatment-free survival: a cohort study. Med Oncol 35(9):121
Timmerman R, McGarry R, Yiannoutsos C et al (2006) Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early stage lung cancer. J Clin Oncol 24:4833–4839
Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K et al (2011) Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 81(5):1352–1358
Figlia V, Mazzola R, Cuccia F, Alongi F, Mortellaro G, Cespuglio D et al (2018) Hypo-fractionated stereotactic radiation therapy for lung malignancies by means of helical tomotherapy: report of feasibility by a single-center experience. Radiol Med 123(6):406–414
Chaudhuri AA, Tang C, Binkley MS, Jin M, Wynne JF, von Eyben R et al (2015) Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung Cancer 89(1):50–56
Chang JY, Li QQ, Xu QY, Allen PK, Rebueno N, Gomez DR et al (2014) Stereotactic ablative radiation therapy for centrally located early stage or isolated parenchymal recurrences of non-small cell lung cancer: how to fly in a “no fly zone”. Int J Radiat Oncol Biol Phys 88(5):1120–1128
Alongi F, Mazzola R, Figlia V, Guckenberger M (2018) Stereotactic body radiotherapy for lung oligometastases: literature review according to PICO criteria. Tumori 104(3):148–156
De Bari B, Filippi AR, Mazzola R, Bonomo P, Trovò M, Livi L, Alongi F (2015) Available evidence on re-irradiation with stereotactic ablative radiotherapy following high-dose previous thoracic radiotherapy for lung malignancies. Cancer Treat Rev 41(6):511–518
Fleckenstein J, Boda-Heggemann J, Siebenlist K, Gudzheva T, Prakofyeva N, Lohr F et al (2018) Non-coplanar VMAT combined with non-uniform dose prescription markedly reduces lung dose in breath-hold lung SBRT. Strahlenther Onkol. https://doi.org/10.1007/s00066-018-1316-0
Brunner TB, Nestle U, Adebahr S, Gkika E, Wiehle R, Baltas D et al (2016) Simultaneous integrated protection: a new concept for high-precision radiation therapy. Strahlenther Onkol 192(12):886–894
Crane CH, Koay EJ (2016) Solutions that enable ablative radiotherapy for large liver tumors: fractionated dose painting, simultaneous integrated protection, motion management, and computed tomography image guidance. Cancer 122(13):1974–1986
Chang JY, Bezjak A, Mornex F, IASLC Advanced Radiation Technology Committee (2015) Stereotactic ablative radiotherapy for centrally located early stage non-small-cell lung cancer: what we have learned. J Thorac Oncol 10(4):577–585
www.nccn.org/professionals/physician_gls/pdf/nscl.pdf accessed on 1 July 2018
Grimm J et al (2011) Dose tolerance limits and dose volume histogram evaluation for stereotactic body radiotherapy. J Appl Clin Med Phys 12(2):267–292
Ikezoe J, Takashima S, Morimoto S, Kadowaki K, Takeuchi N, Yamamoto T et al (1988) CT appearance of acute radiation-induced injury in the lung. AJR Am J Roentgenol 150(4):765–770
Lo SS, Fakiris AJ, Chang EL, Mayr NA, Wang JZ, Papiez L et al (2010) Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol 7(1):44–54
Roesch J, Panje C, Sterzing F, Mantel F, Nestle U, Andratschke N et al (2016) SBRT for centrally localized NSCLC—What is too central? Radiat Oncol 11(1):157
Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J et al (2010) Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 303(11):1070–1076
Timmerman RD, Paulus R, Galvin J, Michalski J, Straube W, Bradley J et al (2009) Stereotactic body radiation therapy for medically inoperable early-stage lung cancer patients: analysis of RTOG 0236. Int J Radiat Oncol Biol Phys 75:S3
Bezjak A, Paulus R, Gaspar LE, Timmerman RD, Straube WL, Ryan WF et al (2016) Primary study endpoint analysis for NRG oncology/RTOG 0813 trial of Stereotactic Body Radiation Therapy (SBRT) for centrally located Non-Small Cell Lung Cancer (NSCLC). Int J Radiat Oncol Biol Phys 94:5–6
Li Q, Swanick CW, Allen PK, Gomez DR, Welsh JW, Liao Z et al (2014) Stereotactic ablative radiotherapy (SABR) using 70 Gy in 10 fractions for non-small cell lung cancer: exploration of clinical indications. Radiother Oncol 112(2):256–261
Haasbeek CJ, Lagerwaard FJ, Slotman BJ, Senan S (2011) Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol 6(12):2036–2043
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. Mazzola, R. Ruggieri, V. Figlia, M. Rigo, N.G. Levra, F. Ricchetti, L. Nicosia, S. Corradini, and F. Alongi declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Mazzola, R., Ruggieri, R., Figlia, V. et al. Stereotactic body radiotherapy of central lung malignancies using a simultaneous integrated protection approach. Strahlenther Onkol 195, 719–724 (2019). https://doi.org/10.1007/s00066-018-01419-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-018-01419-0