Abstract
Purpose
The purpose of this work is to analyze patterns of care and outcome after stereotactic body radiotherapy (SBRT) for centrally located, early-stage, non-small cell lung cancer (NSCLC) and to address the question of potential risk for increased toxicity in this entity.
Methods and materials
A total of 90 patients with centrally located NSCLC were identified among 613 cases in a database of 13 German and Austrian academic radiotherapy centers. The outcome of centrally located NSCLC was compared to that of cases with peripheral tumor location from the same database.
Results
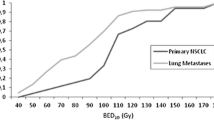
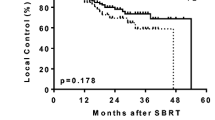
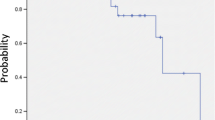
Patients with central tumors most commonly presented with UICC stage IB (50 %), while the majority of peripheral lesions were stage IA (56 %). Average tumor diameters were 3.3 cm (central) and 2.8 cm (peripheral). Staging PET/CT was available for 73 and 74 % of peripheral and central tumors, respectively. Biopsy was performed in 84 % (peripheral) and 88 % (central) of cases. Doses varied significantly between central and peripheral lesions with a median BED10 of 72 Gy and 84 Gy, respectively (p < 0.001). Fractionation differed as well with medians of 5 (central) and 3 (peripheral) fractions (p < 0.001). In the Kaplan–Meier analysis, 3-year actuarial overall survival was 29 % (central) and 51 % (peripheral; p = 0.004) and freedom from local progression was 52 % (central) and 84 % (peripheral; p < 0.001). Toxicity after treatment of central tumors was low with no grade III/IV and one grade V event. Mortality rates were 0 and 1 % after 30 and 60 days, respectively.
Conclusion
Local tumor control in patients treated with SBRT for centrally located, early-stage NSCLC was favorable, provided ablative radiation doses were prescribed. This was, however, not the case in the majority of patients, possibly due to concerns about treatment-related toxicity. Reported toxicity was low, but prospective trials are needed to resolve the existing uncertainties and to establish safe high-dose regimens for this cohort of patients.
Zusammenfassung
Ziel
Ziel dieser Arbeit war die Analyse von Behandlungsmodalitäten und -ergebnissen nach stereotaktischer Körperstrahlentherapie („stereotactic body radiotherapy“, SBRT) bei zentral gelegenem nichtkleinzelligem Lungenkarzinom („non-small cell lung cancer“, NSCLC). Ebenfalls untersucht wurde, ob im Vergleich zu peripheren Tumoren ein erhöhtes Risiko für posttherapeutische Toxizität besteht.
Methoden und Material
Aus 613 Behandlungsfällen einer Datenbank von 13 hochschulassoziierten Strahlentherapiezentren in Deutschland und Österreich wurden insgesamt 90 Patienten mit zentral gelegenen NSCLC identifiziert. Das Outcome der Patienten mit zentralem NSCLC wurde verglichen mit dem von Patienten aus derselben Datenbank mit peripherer Tumorlokalisation.
Ergebnisse
Die meisten zentralen Tumoren waren Karzinome im UICC-Stadium IB (50 %), die Mehrheit der peripher gelegenen Läsionen dagegen befand sich im Stadium IA (56 %). Die durchschnittlichen Tumordurchmesser betrugen 3,3 cm (zentrale) bzw. 2,8 cm (periphere). Staging-PET/CT-Aufnahmen gab es für 73 bzw. 74 % der peripheren bzw. zentralen Tumoren, Biopsien für 84% (periphere) bzw. 88 % (zentrale). Die Bestrahlungsdosen für zentrale und periphere Läsionen unterschieden sich signifikant voneinander, der BED10-Median lag bei 72 bzw. 84 Gy (p < 0,001). Auch die Fraktionierung war unterschiedlich: die Mediane waren 5 (zentral) und 3 (peripher; p < 0,001) Fraktionen. Nach Kaplan-Meier-Analyse lag das aktuarische Dreijahresüberleben bei 29% (zentral) bzw. 51% (peripher; p =0,004), das lokal progressionsfreie Überleben bei 52% (zentral) bzw. 84% (peripher; p <0,001). Die Toxizität nach der Therapie zentraler Tumoren war gering, es gab kein Grad-III/IV- und nur ein Grad-V-Ereignis. Die 30- bzw. 60-Tage-Mortalitätsraten lagen bei 0 bzw. 1%.
Fazit
Die lokale Kontrolle von zentral gelegenen NSCLC im Frühstadium durch SBRT war akzeptabel − unter der Voraussetzung der Verwendung ablativer Strahlendosen. Dies war bei den meisten Patienten allerdings nicht der Fall, möglicherweise wegen Bedenken über behandlungsbedingte Toxizität. Trotz der milden berichteten Toxizität sind prospektive Studien erforderlich, um die bestehenden Unsicherheiten zu beseitigen und sichere Hochdosisregime für Patienten mit zentralem NSCLC zu etablieren.



Similar content being viewed by others
References
Baumann P, Nyman J, Hoyer M et al (2009) Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 27:3290–3296
Onishi H, Shirato H, Nagata Y et al (2011) Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 81:1352–1358
Ricardi U, Filippi AR, Guarneri A et al (2010) Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer 68:72–77
Palma D, Visser O, Lagerwaard FJ et al (2010) Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol 28:5153–5159
Lagerwaard FJ, Verstegen NE, Haasbeek CJA et al (2012) Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 83:348–353
Guckenberger M (2011) What is the current status of stereotactic body radiotherapy for stage I non-small cell lung cancer? J Thorac Dis 3:147–149
Duncker-Rohr V, Nestle U, Momm F et al (2013) Stereotactic ablative radiotherapy for small lung tumors with a moderate dose. Favorable results and low toxicity. Strahlenther Onkol 189:33–40
Timmerman R, Paulus R, Galvin J et al (2010) Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 303:1070–1076
Timmerman R, McGarry R, Yiannoutsos C et al (2006) Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 24:4833–4839
Corradetti MN, Haas AR, Rengan R (2012) Central-airway necrosis after stereotactic body-radiation therapy. N Engl J Med 366:2327–2329
Onimaru R, Shirato H, Shimizu S et al (2003) Tolerance of organs at risk in small-volume, hypofractionated, image-guided radiotherapy for primary and metastatic lung cancers. Int J Radiat Oncol Biol Phys 56:126–135
Song SY, Choi W, Shin SS et al (2009) Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer 66:89–93
Haasbeek CJA, Lagerwaard FJ, Slotman BJ et al (2011) Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol 6:2036–2043
Bral S, Gevaert T, Linthout N et al (2011) Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: results of a Phase II trial. Int J Radiat Oncol Biol Phys 80:1343–1349
Cannon DM, Mehta MP, Adkison JB et al (2013) Dose-limiting toxicity after hypofractionated dose-escalated radiotherapy in non-small-cell lung cancer. J Clin Oncol 31:4343–4348
Oshiro Y, Aruga T, Tsuboi K et al (2010) Stereotactic body radiotherapy for lung tumors at the pulmonary hilum. Strahlenther Onkol 186:274–279
Baba F, Shibamoto Y, Ogino H et al (2010) Clinical outcomes of stereotactic body radiotherapy for stage I non-small cell lung cancer using different doses depending on tumor size. Radiat Oncol 5:81
Senthi S, Haasbeek CJA, Slotman BJ et al (2013) Outcomes of stereotactic ablative radiotherapy for central lung tumours: a systematic review. Radiother Oncol 106:276–282
Nestle U, Faivre-Finn C, Deruysscher D et al (2013) Stereotactic body radiotherapy (SBRT) in central non-small cell lung cancer (NSCLC): solid evidence or ‘no-go’? Radiother Oncol 109:178–179
Guckenberger M, Allgäuer M, Appold S et al (2013) Safety and efficacy of stereotactic body radiotherapy for stage I non-small-cell lung cancer in routine clinical practice: a patterns-of-care and outcome analysis. J Thorac Oncol 8:1050–1058
Schemper M, Smith TL (1996) A note on quantifying follow-up in studies of failure time. Control Clin Trials 17:343–346
Sibley GS, Jamieson TA, Marks LB et al (1998) Radiotherapy alone for medically inoperable stage I non-small-cell lung cancer: the Duke experience. Int J Radiat Oncol Biol Phys 40:149–154
Wulf J, Baier K, Mueller G et al (2005) Dose-response in stereotactic irradiation of lung tumors. Radiother Oncol 77:83–87
Guckenberger M, Wulf J, Mueller G et al (2009) Dose-response relationship for image-guided stereotactic body radiotherapy of pulmonary tumors: relevance of 4D dose calculation. Int J Radiat Oncol Biol Phys 74:47–54
Grills IS, Hope AJ, Guckenberger M et al (2012) A collaborative analysis of stereotactic lung radiotherapy outcomes for early-stage non-small-cell lung cancer using daily online cone-beam computed tomography image-guided radiotherapy. J Thorac Oncol 7:1382–1393
Palma D, Lagerwaard F, Rodrigues G et al (2012) Curative treatment of stage I non-small-cell lung cancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review. Int J Radiat Oncol Biol Phys 82:1149–1156
Fakiris AJ, McGarry RC, Yiannoutsos CT et al (2009) Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 75:677–682
Andratschke N, Zimmermann F, Boehm E et al (2011) Stereotactic radiotherapy of histologically proven inoperable stage I non-small cell lung cancer: patterns of failure. Radiother Oncol 101:245–249
Chang JY, Li Q-Q, Xu Q-Y et al (2014) Stereotactic ablative radiation therapy for centrally located early stage or isolated parenchymal recurrences of non-small cell lung cancer: how to fly in a ‘no fly zone’. Int J Radiat Oncol Biol Phys 88:1120–1128
Compliance with ethical guidelines
Conflict of interest
Dr. Andratschke reports grants from the Bavarian State Ministry of the Environment and Public Health during the conduct of the study and payments for lectures by Accuray Inc., Brainlab AG and Merck Serono. D. Schanne, U. Nestle, M. Allgäuer, S. Appold, U. Dieckmann, I. Ernst, U. Ganswindt, A.L. Grosu, R. Holy, M. Molls, M. Nevinny-Stickel, S. Semrau, F. Sterzing, A. Wittig, and M. Guckenberger state that there are no conflicts of interest.
All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form). Informed consent was obtained from all patients included in studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schanne, D., Nestle, U., Allgäuer, M. et al. Stereotactic body radiotherapy for centrally located stage I NSCLC. Strahlenther Onkol 191, 125–132 (2015). https://doi.org/10.1007/s00066-014-0739-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0739-5
Keywords
- Toxicity
- Non-small cell lung cancer
- Central lung cancer
- Fluorodeoxyglucose F18
- Positron-emission tomography PET