Abstract
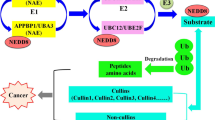
Neddylation modification is frequently overexpressed in many types of human tumors. As a result, targeting neddylation pathway has been identified as viable anticancer therapeutic strategy. The NEDD8-activating enzyme (NAE) serves as a crucial role in a variety of cellular functions. Here, a new library of piperidine analogs was developed, produced and assessed for antiproliferative efficacy against A549, MGC-803, MCF-7KYSE-30 cell lines. The cell-based mechanistic studies showed that IIb-10 bearing the benzoyl hydrazine motif can selectively inhibit the Neddylation modification of Cullin1 and Cullin3 by inhibiting NEDD8 activase and then, leads to a dose-dependent reduction in the level of UBC12-NEDD8 complex via interacting with NAE1 directly. Cellular mechanisms elucidated that compound IIb-10 has the ability to halt the cell cycle of MGC-803 cells at G2/M phase and trigger apoptosis. Altogether, the hydrazide -linked piperidine derivatives may be promising candidates as lead compounds for the development of highly effective neddylation inhibitors.











Similar content being viewed by others
References
Jin L, Williamson A, Banerjee S, Philipp I, Rape M. Mechanism of ubiquitin-chain formation by the human anaphase-promoting complex. Cell. 2008;133:653–65.
Newton K, Matsumoto ML, Wertz IE, Kirkpatrick DS, Lill JR, Tan J, et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell. 2008;134:668–78.
Walczak H, Iwai K, Dikic I. Generation and physiological roles of linear ubiquitin chains. BMC Biol. 2012;10:23.
Wickliffe KE, Williamson A, Meyer HJ, Kelly A, Rape M. K11-linked ubiquitin chains as novel regulators of cell division. Trends Cell Biol. 2011;21:656–63.
Kumar S, Yoshida Y, Noda M. Cloning of a cDNA which encodes a novel ubiquitin-like protein. Biochem. Biochem. Bioph. Res. Co. 1993;195:393–9.
Soucy TA, Smith PG, Milhollen MA, Berger AJ, Langston SP. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–6.
Yoshimura C, Muraoka H, Ochiiwa H, Tsuji S, Hashimoto A, Kazuno H, et al. TAS4464, A Highly Potent and Selective Inhibitor of NEDD8-Activating Enzyme, Suppresses Neddylation and Shows Antitumor Activity in Diverse Cancer Models. Mol. Cancer Thera. 2019;18:1205–16.
Yamamoto N, Shimizu T, Yonemori K, Kitano S, Takahashi S. A first-in-human, phase 1 study of the NEDD8 activating enzyme E1 inhibitor TAS4464 in patients with advanced solid tumors. Investig New Drugs. 2021;39:1036–46.
Lu P, Liu X, Yuan X, He M, Wang Y, Zhang Q, et al. Discovery of a novel NEDD8 Activating Enzyme Inhibitor with Piperidin-4-amine Scaffold by Structure-Based Virtual Screening. Acs Chem Biol. 2016;11:1901–06.
Liu X, Zhang L, Tan JG, Xu HH. Design and synthesis of N-alkyl-N′-substituted 2,4-dioxo-3,4-dihydropyrimidin-1-diacylhydrazine derivatives as ecdysone receptor agonist. Bioorg. Med. Chem. 2013;21:4687–97.
Moosa BA, Ali SA. Regioselective transformation of 6/5-fused bicyclic isoxazolidines to second-generation cyclic aldonitrones. Arkivoc. 2010;10:132–48.
Jain N, Aeluri R, Alla M, et al. Synthesis and antiproliferative activity of imidazo[1,2-a]pyrimidine Mannich bases. Eur. J Med. Chem. 2015;100:18–23.
Zanin L, Jimenez D, Birolli W, Venncio T, Valdes T, Leito A, et al. Synthesis of 1,2,3-triazole Compounds by Click Chemistry in Aqueous Medium and Evaluation of Bactericidal and Antitumoral Properties. Curr Bioactive Compounds. 2022;18:10.
Widler L, Green J, Missbach M, Ua M, Altmann E. 7-Alkyl- and 7-Cycloalkyl-5-aryl-pyrrolo[2,3- d]pyrimidines—potent inhibitors of the tyrosine kinase c-Src. Bioorg. Med. Chem. Lett. 2001;11:849–52.
Garcia J, Mata EG, Tice CM, Hormann RE, Michelotti EL. Evaluation of Solution and Solid-Phase Approaches to the Synthesis of Libraries of α,α-Disubstituted-α-acylaminoketones. J Comb. Chem. 2005;7:843–63.
Wang J, Li F, Pei W, Yang M, Wu Y, Ma D, et al. Selective cleavage of the N-propargyl group from sulfonamides and amides under ruthenium catalysis. Tetrahedron Lett. 2018;59:1902–5.
Kettler K, Sakowski J, Wiesner J, Ortmann R, Jomaa H, Schlitzer M. Novel lead structures for antimalarial farnesyltransferase inhibitors. Pharmazie. 2005;60:323–27.
Xu J, Berastegui-Cabrera J, Ye N, Carretero-Ledesma M, Zhou J. Discovery of Novel Substituted N-(4-Amino-2-chlorophenyl)-5-chloro-2-hydroxybenzamide Analogues as Potent Human Adenovirus Inhibitors. J. Med. Chem. 2020;63:12830–52.
Leipold F, Hussain S, Ghislieri D, Turner NJ. Asymmetric Reduction of Cyclic Imines Catalyzed by a Whole-Cell Biocatalyst Containing an (S)-Imine Reductase. Chemcatchem. 2013;5:3505–8.
Hoque ME, Hassan MMM, Chattopadhyay B. Remarkably Efficient Iridium Catalysts for Directed C(sp 2)–H and C(sp 3)–H Borylation of Diverse Classes of Substrates. J. Am. Chem. Soc. 2021;143:5022–37.
Xu J, Chen J, Gao F, Xie S, Xu X, Jin Z, et al. Sequential Functionalization of meta-C-H and ipso-C-O Bonds of Phenols. J. Am. Chem. Soc. 2019;141:1903–7.
Kumar R, Yar MS, Rai AK, Chaturvedi S. Synthesis and biological evaluation of some novel 1,3,4-oxadiazoles derived from bi phenyl 4-carboxylic acid. Der Pharm Lett. 2013;5:366–70.
Xian J, Wang S, Jiang Y, Li L, Cai L, Chen P, et al. Overexpressed NEDD8 as a potential therapeutic target in esophageal squamous cell carcinoma. 癌症生物学与医学: 英文版. 2022;004:019.
Li JA, Song C, Rong Y, Kuang T, Wang D, Xu X, et al. Chk1 inhibitor SCH 900776 enhances the antitumor activity of MLN4924 on pancreatic cancer. Cell Cycle. 2018;17:191–99.
Zhang Q, Hou D, Luo Z, Chen P, Lv B, Wu L, et al. The novel protective role of P27 in MLN4924-treated gastric cancer cells. Cell Death Dis. 2015;6:e1867.
Li L, Kang J, Zhang W, Cai L, Jia L. Validation of NEDD8-conjugating enzyme UBC12 as a new therapeutic target in lung cancer. EBioMedicine. 2019;45:81–91.
Acknowledgements
This work was supported by Henan science and technology key project (No. 202102310148).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Guan, S., Tian, Z. et al. Identification of novel benzoyl hydrazine derivatives as activators of neddylation pathway to inhibit the tumor progression in vitro. Med Chem Res 33, 504–517 (2024). https://doi.org/10.1007/s00044-024-03193-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-024-03193-4