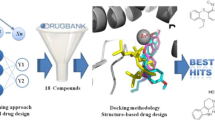
Abstract
Neprilysin (NEP, EC: 3.4.24.11) is an enzymatic membrane protein considered the prototype of the M13 zinc metallopeptidase family. Inhibitors of this enzyme constitute therapeutic targets for cardiovascular diseases. In spite of the existing alternatives for the treatment of these pathologies, the quality of life and the prognosis of the patients remain precarious, so the discovery of new drugs is required for this purpose. In this sense, in silico methods play a fundamental role, since they are an alternative to the traditional “trial and error” methods for obtaining new molecular entities. Taking into account that the main motivation of this work is to propose theoretical models, for the identification of new Neprilysin inhibitor compounds through a virtual screening system, QSAR-LDA and QSAR-MLP models were developed with accuracy >80%. Previously, a threshold was established for the inhibition of NEP using the piecewise regression method (pIC50 = 6.855), and from this value, two classes of compounds (potent inhibitors and poor/moderate inhibitors) were formed. The developed models were used for the virtual screening of compounds, establishing as rules: that they are predicted as active by both models, that they are within the domain of application, and that they possess drug-like properties for oral administration. Following this strategy, eight potential Neprilysin inhibitor compounds were identified, concluding that the proposed computational tools constitute an efficient method for the identification of new inhibitor drugs for this enzyme.







Similar content being viewed by others
References
Adams MB, Bobadilla RV, Blandy SL, Kablaoui FR (2016) Mending the broken heart: a neprilysin inhibitor for heart failure. J Am Acad Nurse Pract 12:111–114
Baldi P, Brunak S, Chauvin Y, Andersen CA, Nielsen H (2000) Assessing the accuracy of prediction algorithms for classification: an overview. Bioinformatics 16:412–424
Barigye SJ, Freitas MP, Ausina P, Zancan P, Sola-Penna M, Castillo-Garit JA (2018) Discrete fourier transform-based multivariate image analysis: application to modeling of aromatase inhibitory activity. ACS Combinatorial Sci 20:75–81
Barrett AJ, Rawlings ND, Woessner JF (2012) Handbook of proteolytic enzymes. Academic Press, Elsevier, London, UK
Bayes-Genis A, Morant-Talamante N, Lupón J (2016) Neprilysin and natriuretic peptide regulation in heart failure. Curr Heart Fail Rep 13:151–157
Canizares-Carmenate Y, Hernandez-Morfa M, Torrens F, Castellano G, Castillo Garit JA (2017) Larvicidal activity prediction against Aedes aegypti mosquito using computational tools. J Vector Borne Dis 54:164–171
Cañizares-Carmenate Y, Mena-Ulecia K, Perera-Sardiña Y, Torrens F, Castillo-Garit JA (2019) An approach to identify new antihypertensive agents using Thermolysin as model: in silico study based on QSARINS and docking. Arab J Chem 12:4861–4877
Castillo-Garit JA, Casañola-Martin GM, Le-Thi-Thu H, Pham-The H, Barigye SJ (2017) A simple method to predict blood-brain barrier permeability of drug- like compounds using classification trees. Med Chem 13:664–669
Castillo-Garit JA, del Toro-Cortés O, Vega MC, Rolón M, Rojas de Arias A, Casañola-Martin GM, Escario JA, Gómez-Barrio A, Marrero-Ponce Y, Torrens F, Abad C (2015) Bond-based bilinear indices for computational discovery of novel trypanosomicidal drug-like compounds through virtual screening. Eur J Med Chem 96:238–244
De Lombaert S, Blanchard L, Tan J, Sakane Y, Berry C, Ghai RD (1995) Non-peptidic inhibitors of Neutral Endopeptidase 24.11. Discovery and optimization of potency. Bioorg Med Chem Lett 5:145–150
Dragon (2017) Dragon (software for molecular descriptor calculation) version 7.0.10. https://chm.kode-solutions.net
De Lombaert S, Erion MD, Tan J, Blanchard L, el-Chehabi L, Ghai RD, Sakane Y, Berry C, Trapani AJ (1994) N-Phosphonomethyl Dipeptides and Their Phosphonate Prodrugs, a New Generation of Neutral Endopeptidase (NEP, EC 3.4.24.11) Inhibitors. J Med Chem 37:498–511
Escario García-Trevijano JA (2016) “Nuevos métodos in sílico y clásicos de selección de moléculas farmacológicamente activasactivas”, lecture given on November 7 at the Real Academia de Ciencias Veterinarias de España, Madrid
Fillion E, Gravel D (1996) Design and synthesis of a new class of conformationally constrained inhibitors to probe the active sites of thermolysine and neutral endopeptidase 24.11. Bioorg Med Chem Lett 6:2097–2102
Fisher RA (1936) The use of multiple measurements in taxonomic problems. Ann Eugen 7:179–188
FitzGerald GA (2011) Re-engineering drug discovery and development. Issue Brief 17:1–4
Glossop MS, Bazin RJ, Dack KN, Fox DNA, MacDonald GA, Mills M, Owen DR, Phillips C, Reeves KA, Ringer TJ, Strang RS, Watson CAL (2011) Synthesis and evaluation of heteroarylalanine diacids as potent and selective neutral endopeptidase inhibitors. Bioorg Med Chem Lett 21:3404–3406
Gómez-Mancebo JR, Arocha JI, Franklin M, Díaz NL (2017) The natriuretic peptide system and neprilysin inhibition in heart failure. Av Cardiol 37:90–100
Gomez-Monterrey I, Beaumont A, Nemecek P, Roques BP, Fournie-Zaluski MC (1994) New thiol inhibitors of neutral endopeptidase EC 3.4.24.1 1: synthesis and enzyme active-site recognition. J Med Chem 37:1865–1873
Gomez-Monterrey I, Turcaud S, Lucas E, Bruetschy L, Roques BP, Fournib-Zaluski MC (1993) Exploration of neutral endopeptidase active site by a series of new thiol-containing inhibitors. J Med Chem 36:87–94
Gramatica P (2007) Principles of QSAR models validation: internal and external. QSAR Comb Sci 26:694–701
Gramatica P, Chirico N, Papa E, Cassani S, Kovarich S (2013) QSARINS: a new software for the development, analysis, and validation of QSAR MLR models. J Comput Chem 34:2121–2132
Hallinan JS (2012) Data mining for microbiologists. In: Methods Microbiol. Elsevier Ltd, vol 39, pp 27–79
Hernandez JF, Soleilhac JM, Roques BP, Fourni-Zaluski MC (1988) Retro-inverso concept applied to the complete inhibitors of enkephalin-degrading enzymes. J Med Chem 31:1825–1831
James K, Palmer MJ (1993) Gem-cycloalkyl substituted thiol inhibitors of Neutral Endopeptiddase 24.11. Synthesis via nucleophilic opening of 2,2-spiro-β-lactones. Bioorg Med Chem Lett 3:825–830
Jaworska J, Nikolova-Jeliazkova N (2007) How can structural similarity analysis help in category formation? SAR QSAR Environ Res 18:195–207
Ksander GM, de Jesus R, Yuan A, Ghai RD, Trapani A, McMartin C, Bohacek R (1997) Ortho-substituted benzofused macrocyclic lactams as zinc metalloprotease inhibitors. J Med Chem 40:495–505
Ksander GM, Ghai RD, de Jesus R, Diefenbacher CG, Yuan A, Berry C, Sakane Y, Trapani A (1995) Dicarboxylic acid dipeptide neutral endopeptidase inhibitors. J Med Chem 38:1689–1700
Le-Thi-Thu H, Canizares-Carmenate Y, Marrero-Ponce Y, Torrens F, Castillo-Garit JA (2016) Prediction of Caco-2 cell permeability using bilinear indices and multiple linear regression. Lett Drug Des Discov 13:161–169
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46:3–26
Lippmann RP (1988) An introduction to computing with neural nets. ACM SIGARCH Comput Arch N. 16:7–25
Llorens C, Gacel G, Swerts JP, Perdrisot R, Fournie-Zaluski MC, Schwartz JC, Roques BP (1980) Rational design of enkephalinase inhibitors: substrate specificity of enkephalinase studied from inhibitory potency of various dipeptides. Biochem Biophys Res Commun 96:1710–1716
MacPherson LJ, Bayburt EK, Capparelli MP, Bohacek RS, Clarke FH, Ghai RD, Sakane Y, Berry CJ, Peppard JV, Trapani AJ (1993) Design and Synthesis of an Orally Active Macrocyclic Neutral Endopeptidase 24.11 Inhibitor. J Med Chem 36:3821–3828
Mangiafico S, Costello-Boerrigter LC, Andersen IA, Cataliotti A, Burnett JCJ (2013) Neutral endopeptidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeutics. Eur Heart J 34:886–893
Manzur F, Villarreal T, Moneriz C (2013) Dual inhibition of neprilysin and angiotensin II receptor: promising new strategy in the treatment of cardiovascular disease. Rev Colomb Cardiol 20:386–393
Massart DL, Vandeginste BGM, Buydens LMC, De Jong S, Lewi PJ, Smeyers-Verbeke J (2003) Handbook of chemometrics and qualimetrics. ELSEVIER SCIENCE B. V. Sara Burgerhartstraat, Amsterdam
Mauri A, Consonni V, Pavan M, Todeschini R (2006) DRAGON Software: An easy approach to molecular descriptor calculations. MATCH Commun. Math Comput Chem 56:237–248
Misawa K, Suzuki Y, Takahashi S, Yoshimori A, Takasawa R, Shibuya Y, Tanuma S (2011) Structure-based design of dipeptide derivatives for the human neutral endopeptidase. Bioorg Med Chem 19:5935–5947
Mohanty N, Lee-St. John A, Manmatha R, Rath TM (2013) Shape-Based Image Classification and Retrieval. Handb Stat, Elsevier B V 31:249–267
Oefner C, D’Arcy A, Hennig M, Winkler FK, Dale GE (2000) Structure of Human Neutral Endopeptidase (Neprilysin) Complexed with Phosphoramidon. J Mol Biol 296:341–349
Oosterbaan RJ (1994) Frequency and regression analysis. In: Drainage principles and applications. International Institute for Land Reclamation and Improvement (ILRI), Wageningen, The Netherlands, Publication 16, p 175–224
Poras H, Patouret R, Leiris S, Ouimet T, Fournié-Zaluski MC, Roques BP (2017) Substituted alpha-mercaptoketones, new types of specific neprilysin inhibitors. Bioorg Med Chem Lett 27:3883–3890
Pryde DC, Cook AS, Burring DJ, Jones LH, Foll S, Platts MY, Sanderson V, Corless M, Stobie A, Middleton DS, Foster L, Barker L, Van Der Graaf P, Stacey P, Kohl C, Coggonc S, Beaumont K (2006) Novel selective inhibitors of neutral endopeptidase for the treatment of female sexual arousal disorder. Bioorg Med Chem 15:142–159
Roy K, Kar S, Narayan-Das R (2015) Selected statistical methods in QSAR. In: Understanding the basics of QSAR for applications in pharmaceutical sciences and risk assessment, Elsevier, Boston, USA, p 191–229
Sahli S, Frank B, Schweizer WB, Diederich F, Blum-Kaelin D, Aebi JD, Bohm HJ, Oefner C, Dale GE (2005) Second-generation inhibitors for the metalloprotease neprilysin based on bicyclic heteroaromatic scaffold: synthesis, biological activity, and x-ray crystal-structure analysis. Helv Chim Acta 88:731–750
Sahli S, Stump B, Welti T, Schweizer WB, Diederich F, Blum-Kaelin D, Aebi JD, Böhm HJ (2005) A new class of inhibitors for the metalloprotease neprilysin based on a central imidazole scaffold. Helv Chim Acta 88:707–730
Stanton JL, Ksander GM, de Jesus R, Sperbeck DM (1994) The effect of heteroatom substitution on a series of phosphonate inhibitors of neutral endopeptidase 24.11. Bioorg Med Chem Lett 4:539–542
StatSoft, Inc. (2001) Statistica, version 6 www.statsoft.com, Tulsa
Tamimi NA, Ellis P (2009) Drug development: from concept to marketing! Nephron Clin Pract 113:c125–131
WHO (2018) “Noncommunicable diseases”. https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases
WHO (2019) World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health 7:e1332–e1345
Worth AP, Bassan A, De Bruijn J, Gallegos-Saliner A, Netzeva T, Patlewicz G, Pavan M, Tsakovska I, Eisenreich S (2007) The role of the European Chemicals Bureau in promoting the regulatory use of (Q)SAR methods. SAR QSAR Environ Res 18:111–125
Xie J, Soleilhac JM, Schmidt C, Peyroux J, Roques BP, Fourni-Zaluski MC (1989) New Kelatorphan-Related Inhibitors of Enkephalin Metabolism: Improved Antinociceptive Properties. J Med Chem 32:1497–1503
Zupan J, Gasteiger J (1999) Neural Networks for Chemistry and Drug Design. Wiley-VCH Publishers, Weinheim (Germany)
Acknowledgements
One of the authors, FT, thanks support from Universidad Católica de Valencia San Vicente Mártir (Project No. 2019-217-001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cañizares-Carmenate, Y., Alcántara Cárdenas, A., Roche Llerena, V. et al. Computational approach to the discovery of potential neprilysin inhibitors compounds for cardiovascular diseases treatment. Med Chem Res 29, 897–909 (2020). https://doi.org/10.1007/s00044-020-02529-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02529-0