Abstract
Sumoylation is a reversible post-translational modification essential to the modulation of neuronal function, including neurotransmitter release and synaptic plasticity. A tightly regulated equilibrium between the sumoylation and desumoylation processes is critical to the brain function and its disruption has been associated with several neurological disorders. This sumoylation/desumoylation balance is governed by the activity of the sole SUMO-conjugating enzyme Ubc9 and a group of desumoylases called SENPs, respectively. We previously demonstrated that the activation of type 5 metabotropic glutamate receptors (mGlu5R) triggers the transient trapping of Ubc9 in dendritic spines, leading to a rapid increase in the overall synaptic sumoylation. However, the mechanisms balancing this increased synaptic sumoylation are still not known. Here, we examined the diffusion properties of the SENP1 enzyme using a combination of advanced biochemical approaches and restricted photobleaching/photoconversion of individual hippocampal spines. We demonstrated that the activation of mGlu5R leads to a time-dependent decrease in the exit rate of SENP1 from dendritic spines. The resulting post-synaptic accumulation of SENP1 restores synaptic sumoylation to initial levels. Altogether, our findings reveal the mGlu5R system as a central activity-dependent mechanism to maintaining the homeostasis of sumoylation at the mammalian synapse.







Similar content being viewed by others
References
Mabb AM, Ehlers MD (2010) Ubiquitination in postsynaptic function and plasticity. Annu Rev Cell Dev Biol 26:179–210
Schorova L, Martin S (2016) Sumoylation in synaptic function and dysfunction. Front Synaptic Neurosci 8:9
Henley JM, Carmichael RE, Wilkinson KA (2018) Extranuclear SUMOylation in neurons. Trends Neurosci 41(4):198–210
Girach F, Craig TJ, Rocca DL, Henley JM (2013) RIM1alpha SUMOylation is required for fast synaptic vesicle exocytosis. Cell Rep 5(5):1294–1301
Loriol C, Casse F, Khayachi A, Poupon G, Chafai M, Deval E, Gwizdek C, Martin S (2014) mGlu5 receptors regulate synaptic sumoylation via a transient PKC-dependent diffusional trapping of Ubc9 into spines. Nat Commun 5:5113
Craig TJ, Anderson D, Evans AJ, Girach F, Henley JM (2015) SUMOylation of Syntaxin1A regulates presynaptic endocytosis. Sci Rep 5:17669
Khayachi A, Gwizdek C, Poupon G, Alcor D, Chafai M, Casse F, Maurin T, Prieto M, Folci A, De Graeve F, Castagnola S, Gautier R, Schorova L, Loriol C, Pronot M, Besse F, Brau F, Deval E, Bardoni B, Martin S (2018) Sumoylation regulates FMRP-mediated dendritic spine elimination and maturation. Nat Commun 9(1):757
Martin S, Nishimune A, Mellor JR, Henley JM (2007) SUMOylation regulates kainate-receptor-mediated synaptic transmission. Nature 447(7142):321–325
Craig TJ, Jaafari N, Petrovic MM, Rubin PP, Mellor JR, Henley JM (2012) Homeostatic synaptic scaling is regulated by protein SUMOylation. J Biol Chem 287(27):22781–22788
Chamberlain SE, Gonzalez-Gonzalez IM, Wilkinson KA, Konopacki FA, Kantamneni S, Henley JM, Mellor JR (2012) SUMOylation and phosphorylation of GluK2 regulate kainate receptor trafficking and synaptic plasticity. Nat Neurosci 15(6):845–852
Matunis MJ, Coutavas E, Blobel G (1996) A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J Cell Biol 135(6):1457–1470
Mahajan R, Delphin C, Guan T, Gerace L, Melchior F (1997) A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 88(1):97–107
Nayak A, Muller S (2014) SUMO-specific proteases/isopeptidases: SENPs and beyond. Genome Biol 15(7):422
Loriol C, Parisot J, Poupon G, Gwizdek C, Martin S (2012) Developmental regulation and spatiotemporal redistribution of the sumoylation machinery in the rat central nervous system. PLoS One 7(3):e33757
Loriol C, Khayachi A, Poupon G, Gwizdek C, Martin S (2013) Activity-dependent regulation of the sumoylation machinery in rat hippocampal neurons. Biol Cell 105(1):30–45
Kerscher O (2007) SUMO junction-what’s your function? New insights through SUMO-interacting motifs. EMBO Rep 8(6):550–555
Meulmeester E, Melchior F (2008) Cell biology: SUMO. Nature 452(7188):709–711
Hickey CM, Wilson NR, Hochstrasser M (2012) Function and regulation of SUMO proteases. Nat Rev Mol Cell Biol 13(12):755–766
Gurskaya NG, Verkhusha VV, Shcheglov AS, Staroverov DB, Chepurnykh TV, Fradkov AF, Lukyanov S, Lukyanov KA (2006) Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat Biotechnol 24(4):461–465
Chudakov DM, Lukyanov S, Lukyanov KA (2007) Using photoactivatable fluorescent protein Dendra2 to track protein movement. Biotechniques 42(5):553–557
Casse F, Martin S (2015) Tracking the activity-dependent diffusion of synaptic proteins using restricted photoconversion of Dendra2. Front Cell Neurosci 9:367
Gardoni F, Mauceri D, Malinverno M, Polli F, Costa C, Tozzi A, Siliquini S, Picconi B, Cattabeni F, Calabresi P, Di Luca M (2009) Decreased NR2B subunit synaptic levels cause impaired long-term potentiation but not long-term depression. J Neurosci 29(3):669–677
Gardoni F, Pagliardini S, Setola V, Bassanini S, Cattabeni F, Battaglia G, Di Luca M (2003) The NMDA receptor complex is altered in an animal model of human cerebral heterotopia. J Neuropathol Exp Neurol 62(6):662–675
Thalhammer A, Rudhard Y, Tigaret CM, Volynski KE, Rusakov DA, Schoepfer R (2006) CaMKII translocation requires local NMDA receptor-mediated Ca2+ signaling. EMBO J 25(24):5873–5883
Lemieux M, Labrecque S, Tardif C, Labrie-Dion E, Lebel E, De Koninck P (2012) Translocation of CaMKII to dendritic microtubules supports the plasticity of local synapses. J Cell Biol 198(6):1055–1073
Sun H, Lu L, Zuo Y, Wang Y, Jiao Y, Zeng WZ, Huang C, Zhu MX, Zamponi GW, Zhou T, Xu TL, Cheng J, Li Y (2014) Kainate receptor activation induces glycine receptor endocytosis through PKC deSUMOylation. Nat Commun 5:4980
Bailey D, O’Hare P (2004) Characterization of the localization and proteolytic activity of the SUMO-specific protease, SENP1. J Biol Chem 279(1):692–703
Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP (2008) A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA 105(31):10762–10767
Wei B, Huang C, Liu B, Wang Y, Xia N, Fan Q, Chen GQ, Cheng J (2018) Mitotic phosphorylation of SENP3 regulates DeSUMOylation of chromosome-associated proteins and chromosome stability. Cancer Res 78(9):2171–2178
Fu J, Yu HM, Chiu SY, Mirando AJ, Maruyama EO, Cheng JG, Hsu W (2014) Disruption of SUMO-specific protease 2 induces mitochondria mediated neurodegeneration. PLoS Genet 10(10):e1004579
Guo C, Hildick KL, Luo J, Dearden L, Wilkinson KA, Henley JM (2013) SENP3-mediated deSUMOylation of dynamin-related protein 1 promotes cell death following ischaemia. EMBO J 32(11):1514–1528
Wang H, Zhuo M (2012) Group I metabotropic glutamate receptor-mediated gene transcription and implications for synaptic plasticity and diseases. Front Pharmacol 3:189
Li X, Luo Y, Yu L, Lin Y, Luo D, Zhang H, He Y, Kim YO, Kim Y, Tang S, Min W (2008) SENP1 mediates TNF-induced desumoylation and cytoplasmic translocation of HIPK1 to enhance ASK1-dependent apoptosis. Cell Death Differ 15(4):739–750
Xiong H, Casse F, Zhou Y, Zhou M, Xiong ZQ, Joels M, Martin S, Krugers HJ (2015) mTOR is essential for corticosteroid effects on hippocampal AMPA receptor function and fear memory. Learn Mem 22(12):577–583
Xiong H, Casse F, Zhou M, Xiong ZQ, Joels M, Martin S, Krugers HJ (2016) Interactions between N-ethylmaleimide-sensitive factor and GluA2 contribute to effects of glucocorticoid hormones on AMPA receptor function in the rodent hippocampus. Hippocampus 26(7):848–856
Acknowledgements
We thank Dr Wang Min for sharing the GFP-SENP1 construct. We gratefully acknowledge the ‘Agence Nationale de la Recherche (Fr)’ (ANR-15-CE16-0015-01) and the Bettencourt-Schueller foundation for financial support. We also thank the French Government for the ‘Investments for the Future’ Laboratory of Excellence ‘SIGNALIFE’ (ANR-11-LABX-0028-01) and the ‘Conseil Régional Provence-Alpes-Côte d’Azur’ for the ‘Microscopy and Imaging Côte d’Azur’ platform funding. LS and MPri are fellows from the international Ph.D. ‘SIGNALIFE’ program.
Author information
Authors and Affiliations
Contributions
LS and MP performed all the live-imaging experiments. LS, MP and AK performed biochemical experiments. GP and MPri prepared viral particles. AF, MP and LS performed the immunocytochemistry. LS, MP, AF and GP prepared neuronal cultures. FC performed initial FRAP experiments. FBr provided computational tools to analyze imaging data. LS, MP, CG and SM contributed to hypothesis development, experimental design and data interpretation. SM provided the overall supervision, the funding and wrote the original draft. All authors discussed the data and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig.
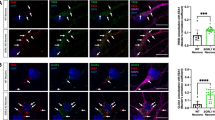
1 GFP-SENP1 is distributed in the nucleus, dendrites and spines and is catalytically active (a) Representative confocal image of a 19 DIV rat hippocampal neuron expressing WT GFP-SENP1 (green) and immunolabelled for PSD-95 (red). (b) Representative confocal image of a 19 DIV rat hippocampal neuron immunoreactive for SENP1 (green) and the synaptic marker PSD-95 (red). Dashed circle indicates the nuclear envelope. Scale bar = 20 µm. (c) Representative image of a 19 DIV rat hippocampal neuron expressing WT GFP-SENP1 (green) and immunolabelled for the post-synaptic marker PSD-95 (red). Scale bars: Left, 20 μm; Right, 2 μm. (d) Graphical representation indicates the percentage of GFP-SENP1 localization in PSD-95 positive compartment (16.9 ± 0.91%) of secondary dendrites (n = 7 neurons). (e) Representative images of a 19 DIV rat hippocampal neuron immunolabelled for the dendritic marker MAP2 (blue), PSD-95 (red) and SENP1 (green). Scale bars: Left, 20 μm; Right, 2 μm. (f) Graphical representation indicates the percentage of endogenous SENP1 staining in PSD-95 positive spines (12.7 ± 0.4%) in secondary dendrites (n = 26 neurons). (g) Representative immunoblot of SUMO1-modified protein levels upon expression of GFP, WT GFP-SENP1 and the catalytically inactive GFP-SENP1 mutant (C603S) in COS7 cells. (h) Representative immunoblots for SENP1, GFP and β-actin in the indicated transfected conditions. (i) Quantitative representation of SUMO1-ylation levels normalized to control GFP ± SEM (GFP-SENP1 WT [63.2 ± 5.8%] and GFP-SENP1 C603S [113.4 ± 6.8%]) from 4 independent experiments. Statistics: One-way ANOVA with a Tukey post-hoc test. P-values are indicated. Supplementary Fig. 2 The synaptic redistribution of SENP1 into spines does not rely on its catalytic activity (a) FRAP curves showing mean values ± SEM of fluorescence intensity of bleached spines for WT and C603S GFP-SENP1 in control (light and dark blue) and bicuculline (red and orange, 25-50 min of sustained bic treatment) conditions. It should be noted that FRAP curves and histograms for WT GFP-SENP1 are taken from Fig. 3c-f, for comparison. (b) FRAP measurement ± SEM: mobile fraction (WT: ctrl [74.5 ± 1.1%], and bic 25-50 min [56.2 ± 1.8%]; C603S: ctrl [73.9 ± 1%], bic [54.2 ± 2.6%]); (c) half-time recovery (t1/2, WT: ctrl [20.79 ± 1 s], bic 25-50 min [33.58 +/- 1.6 s]; C603S: ctrl [27.4 ± 1.2 s], bic [36.8 ± 3.6 s]); and (d) diffusion coefficient (WT: ctrl [0.0135 ± 0.0007 µm2/s], bic 25-50 min [0.0087 ± 0.0007 µm2/s]; C603S: ctrl [0.0092 ± 0.0004 µm2/s], bic [0.0084 ± 0.001 µm2/s]). Spine number WT: ctrl= 164 and bic 25-50 min = 139; C603S: ctrl = 160 and bic 25-50 min = 59, from at least 5 different cultures for WT and 2 independent cultures for C603S GFP-SENP1. Statistics: T1/2 and diff. coef. were analyzed with Mann-Whitney t-test and Fm with parametric t-test. P-values are indicated. Supplementary Fig. 3 Full gel related to the indicated figures. Orange-dashed boxes represent the portion of the image used in figures. (PDF 2459 kb)
Supplementary video 1
Time-lapse imaging (25 Hz) of GFP-SENP1 showing the redistribution of the desumoylation enzymes into spines in basal condition and upon neuronal activation with bicuculline. (AVI 1353 kb)
Supplementary video 2
Comparative time-lapse imaging (15 Hz) of the synaptic GFP-SENP1 Fluorescence Recovery After Photobleaching (FRAP) in basal and bicuculline (15 and 40 min)-treated conditions. (AVI 4547 kb)
Supplementary video 3
Comparative time-lapse imaging (20 Hz) of the synaptic exit of the red-photoconverted Dendra2-SENP1 fluorescence in basal and sustained bicuculline-treated neurons. (AVI 6136 kb)
Rights and permissions
About this article
Cite this article
Schorova, L., Pronot, M., Poupon, G. et al. The synaptic balance between sumoylation and desumoylation is maintained by the activation of metabotropic mGlu5 receptors. Cell. Mol. Life Sci. 76, 3019–3031 (2019). https://doi.org/10.1007/s00018-019-03075-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03075-8